| Title |
Homonymous Hemianopia Due to ErdheimChester Disease |
| Creator |
Hills, William L; Nassef, Ahmad H; Grafe, Marjorie R; Weissman, Jane L; Moster, Stephen J; Falardeau, Julie; Mardekian, Stacey K; Curtis, Mark T; Moster, Mark L |
| Affiliation |
Department of Neuro-Ophthalmology (WLH, JF), Casey Eye Institute, Oregon Health & Science University, Portland, Oregon; Departments of Neurology (WLH) and Ophthalmology (WLH, JF), Oregon Health & Science University, Portland, Oregon; Arab Organization for Industrialization Hospital (AN), Cairo, Egypt; Department of Neuropathology (MRG), Oregon Health & Science University, Portland, Oregon; University of Pennsylvania School of Medicine (SJM), Philadelphia, Pennsylvania; Department of Radiology, Ophthalmology, and Otolaryngology (JLW), Oregon Health & Science University, Portland, Oregon; Department of Pathology (SKM, MTC), Thomas Jefferson University School of Medicine, Philadelphia, Pennsylvania; Department of Neuro-Ophthalmology (MLM), Wills Eye Institute, Philadelphia, Pennsylvania; and Departments of Neurology (MLM) and Ophthalmology (MLM), Thomas Jefferson University School of Medicine, Philadelphia, Pennsylvania |
| Abstract |
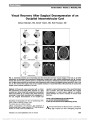
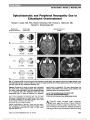
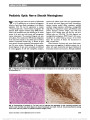
Erdheim-Chester disease (ECD) is a rare non-Langerhans cell histiocytosis typically affecting multiple organ systems. We report 2 patients who presented with homonymous hemianopia and were ultimately diagnosed with biopsy-confirmed ECD. We review the spectrum of ECD and its treatment as well as histopathological and immunohistochemical differentiation from other histiocytic disorders. |
| Subject |
Adult; Antigens, CD; Antigens, CD44; Antigens, Differentiation, Myelomonocytic; Brain; Erdheim-Chester Disease; Extracellular Matrix Proteins; Female; Hemianopsia; Humans; Magnetic Resonance Imaging; Male; Middle Older people; Visual Fields |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227605 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6kh3tdd/227605 |