| Title |
Stereotactic Radiosurgery in the Treatment of a Dural Carotid-Cavernous Fistula |
| Creator |
Chong, Gabriel T; Mukundan, Srinivasan; Kirkpatrick, John P; Zomorodi, Ali; Sampson, John H; Bhatti, MT |
| Affiliation |
Department of Ophthalmology, Duke University Eye Center |
| Abstract |
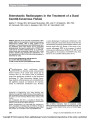
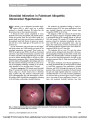
Because of the success of stereotactic radiosurgery (SRS) in the treatment of cerebral arteriovenous malformations (AVMs), SRS is being applied to the treatment of carotid-cavernous dural arteriovenous fistulas (CCDAVFs) when these lesions are not accessible endovascularly. We report a patient with a CCDAVF that could not be accessed endovascularly on 2 attempts, whose fistula was successfully closed with SRS, a less invasive modality than endovascular embolization. Further experience with SRS in this role will be necessary to determine its utility. |
| Subject |
Adult; Ataxia/etiology; Cerebral Aqueduct/abnormalities; Cerebral Aqueduct/pathology; Cerebral Aqueduct/physiopathology; Chronic Disease; Diagnosis, Differential; Disease Progression; Facial Nerve Diseases/etiology; Humans; Magnetic Resonance Imaging; Male; Mesencephalon/abnormalities; esencephalon/pathology; Mesencephalon/physiopathology; Multiple Sclerosis/diagnosis; Multiple Sclerosis/physiopathology; Nervous System Malformations/complications; Nervous System Malformations/pathology; Nervous System Malformations/physiopathology; Neural Pathways/pathology; Neural Pathways/physiopathology; Ocular Motility Disorders/etiology; Syringomyelia/pathology; Syringomyelia/physiopathology |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227059 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6k109bb/227059 |