| Title |
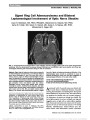
Homonymous Ganglion Cell Layer Thinning After Isolated Occipital Lesion: Macular OCT Demonstrates Transsynaptic Retrograde Retinal Degeneration |
| Creator |
Paolo G. Meier; Philippe Maeder; Randy H. Kardon; Franois-Xavier Borruat |
| Affiliation |
Neuro-Ophthalmology Unit, Hpital Ophtalmique Jules-Gonin (PGM, F-XB), University of Lausanne, Lausanne, Switzerland; Department of Radiology (PGM), CHUV, University of Lausanne, Lausanne, Switzerland; and Department of Veterans Affairs Medical Center (RHK), University of Iowa Hospitals and Clinics, Iowa City, Iowa |
| Abstract |
A 48-year-old man was examined 24 months after medial and surgical treatment of an isolated well-circumscribed right occipital lobe abscess. An asymptomatic residual left homonymous inferior scotoma was present. Fundus examination revealed temporal pallor of both optic discs, and optical coherence tomography (OCT) revealed mild temporal loss of retinal nerve fiber layer in both eyes. No relative afferent pupillary defect was present. Assessment of the retinal ganglion cell layer demonstrated homonymous thinning in a pattern corresponding to the homonymous visual field loss. There were no abnormalities of the lateral geniculate nuclei or optic tracts on review of the initial brain computed tomography and follow-up magnetic resonance imaging. We believe our patient showed evidence of transsynaptic retrograde degeneration after an isolated right occipital lobe lesion, and the homonymous neuronal loss was detected on OCT by assessing the retinal ganglion cell layer. |
| Subject |
Anti-Inflammatory Agents; Brain Injuries; Humans; Magnetic Resonance Imaging; Male; Methylprednisolone; Middle Older people; Nerve Fibers; Occipital Lobe; Retinal Degeneration; Retinal Ganglion Cells; Tomography, Optical Coherence; Tomography, X-Ray Computed; Visual Pathways |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227703 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s66b08p3/227703 |