| Title |
Vogt-Koyangi-Harada Syndrome in a Case of Multiple Sclerosis |
| Creator |
Montero, JA; Sanchis, ME; Fernandez-Munoz, M |
| Affiliation |
VISSUM, Instituto Oftalmológico de Alicante, Retina Unit, Alicante, Spain. msm02va@wanadoo.es |
| Abstract |
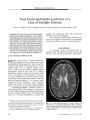
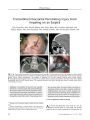
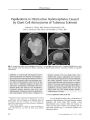
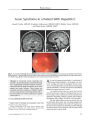
A 34-year-old woman in whom multiple sclerosis (MS) had been diagnosed 2 years earlier developed progressive bilateral visual loss associated with peripapillary exudative retinal detachment and other features of Vogt-Koyanagi-Harada (VKH) syndrome. She was treated with corticosteroid pulses and interferon beta-1A with visual acuity improvement and resolution of the retinal detachment. This is the first reported case of VKH syndrome in a patient with MS. The combination of VKH syndrome and MS suggests a common autoimmune pathogenesis. |
| Subject |
Adjuvants, Immunologic, administration & dosage; Adjuvants, Immunologic, therapeutic use; Adult; Diagnosis, Differential; Disease Progression; Drug Administration Routes; Drug Therapy, Combination; Female; Fluorescein Angiography; Fundus Oculi; Glucocorticoids, administration & dosage; Glucocorticoids, therapeutic use; Humans; Interferon-beta, administration & dosage; Interferon-beta, therapeutic use; Magnetic Resonance Imaging; Multiple Sclerosis, complications; Multiple Sclerosis, diagnosis; Tomography, Optical Coherence; Uveomeningoencephalitic Syndrome, diagnosis; Uveomeningoencephalitic Syndrome, drug therapy; Uveomeningoencephalitic Syndrome, etiology; Visual Acuity |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225627 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6477h01/225627 |