| Title |
Posner-Schlossman Syndrome and Nonarteritic Anterior Ischemic Optic Neuropathy |
| Creator |
Irak, I; Katz, BJ; Zabriskie, NA; Zimmerman, PL |
| Affiliation |
Department of Ophthalmology and Visual Sciences and the John A Moran Eye Center, University of Utah Health Sciences Center, Salt Lake City, Utah, USA. |
| Abstract |
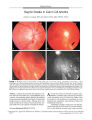
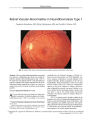
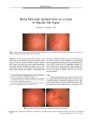
A 41-year-old woman with acute OD pain and decreased visual acuity presented with anterior uveitis, an intraocular pressure of 56 mm Hg, an open angle, ipsilateral nerve fiber bundle visual field defects, and optic nerve edema. With control of intraocular pressure and uveitis, visual acuity improved to 20/25, visual field defects persisted, and optic disc pallor developed. She has remained stable over 23 months of follow-up. This case represents a concurrence of glaucomatocyclitic crisis (Posner-Schlossman syndrome, PSS) and nonarteritic ischemic optic neuropathy (NAION). Although this combination occurs rarely, patients with PSS and other risk factors for NAION, including an optic disc that lacks a physiologic cup, should be protected against NAION by prophylactic treatment with ocular antihypertensive medications. |
| Subject |
Adult; Ciliary Body; Female; Glaucoma/complications; Humans; Intraocular Pressure/drug effects; Optic Disk/pathology; Optic Neuropathy, Ischemic/drug therapy; Optic Neuropathy, Ischemic/etiology; Optic Neuropathy, Ischemic/pathology; Optic Neuropathy, Ischemic/physiopathology; Prostaglandins F, Synthetic/therapeutic use; Syndrome; Uveitis/complications; Visual Acuity; Visual Fields |
| Date |
1994-06 |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225313 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s62g0thm/225313 |