| Title |
Prediction of NOx Emissions from Oxygen-Enriched Burners |
| Creator |
Missaghi, M.; Pourkashanian, M.; Williams, A.; Yap, T. L. |
| Publisher |
University of Utah |
| Date |
1991 |
| Spatial Coverage |
presented at Honolulu, Hawaii |
| Abstract |
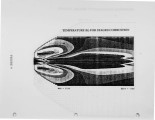
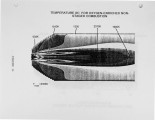
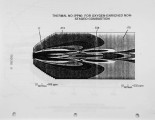
Nitrogen oxides are important air pollutants, believed to play a central role in the formation of photo-chemical smog and acid rain. The' nitrogen oxides are generated in high-temperature combustion processes and thus are released by stationary combustion sources, motor vehicles, as well as a wide range of domestic fossil fuel fired appliances. Recently, the emissions from these sources are legislated. Meeting the ever evolving standards continues to challenge available combustion technology. As a result, the interest in predicting the formation of nitrogen oxides in practical combustion systems has become large. Unfortunately, the formulation of reliable, accurate models has been severely hampered by the absence of proper descriptions of, amongst others, turbulence-chemistry interactions, radical super equilibria, prompt, and fuel-NO chemistry. |
| Type |
Text |
| Format |
application/pdf |
| Language |
eng |
| Rights |
This material may be protected by copyright. Permission required for use in any form. For further information please contact the American Flame Research Committee. |
| Conversion Specifications |
Original scanned with Canon EOS-1Ds Mark II, 16.7 megapixel digital camera and saved as 400 ppi uncompressed TIFF, 16 bit depth. |
| Scanning Technician |
Cliodhna Davis |
| ARK |
ark:/87278/s69c7105 |
| Setname |
uu_afrc |
| ID |
7503 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s69c7105 |