| Title |
Experimental Observations and a Postulated Mechanism of Relase of Iron During the Combustion of Illinois #6 Coal |
| Creator |
Baxter, Larry L.; Mitchell, Reginald E. |
| Publisher |
University of Utah |
| Date |
1988 |
| Spatial Coverage |
presented at Pittsburgh, Pennsylvania |
| Abstract |
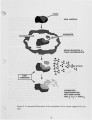
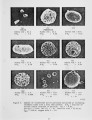
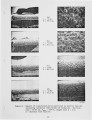
A series of investigations of iron release during the combustion of illinois #6 coal are reported. Experimental measurements are described in which 40-70 percent of the iron in the coal is released within a 25 ms period immediately following coal devolatilization. This iron release dominates the evolution of all other elements in this coal (excluding sulfur) on a mass basis. The release is consistently observed under realistic combustion environments over a particle size range from 75-125 J.Lm and a gas oxygen concentration range of 6-12 percent. Statistical uncertainty of the results is also reported, but is small compared to the measured effect. Further experiments and analysis were conducted to determine the mechanism of iron release. Forms of iron with sufficient volatility to explain the results as a vaporization process were investigated. None was found which could reasonably be expected to form under the experimental conditions. Further experimentation suggests that the mechanism is related to the chemical reactivity of the iron species with oxygen and that the release proceeds through the chemical formation of an iron fume. |
| Type |
Text |
| Format |
application/pdf |
| Language |
eng |
| Rights |
This material may be protected by copyright. Permission required for use in any form. For further information please contact the American Flame Research Committee. |
| Conversion Specifications |
Original scanned with Canon EOS-1Ds Mark II, 16.7 megapixel digital camera and saved as 400 ppi uncompressed TIFF, 16 bit depth. |
| Scanning Technician |
Cliodhna Davis |
| ARK |
ark:/87278/s6nc63rk |
| Setname |
uu_afrc |
| ID |
4888 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6nc63rk |