| Title |
Ideal Combustion of Solid Fuels |
| Creator |
Ling, S. C.; Pao, H. P. |
| Publisher |
University of Utah |
| Date |
1988 |
| Spatial Coverage |
presented at Pittsburgh, Pennsylvania |
| Abstract |
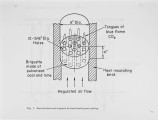
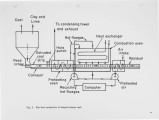
Unlike the unstable. runaway. combustion of micro-fuel particles, solid fuel in the reconstituted form of a specifically shaped charge was found to have stable and ideal combustion characteristics suitable for the automatic control of combustion rate and temperature. Consequently most environmental and operational problems associated with the atomized-combustion process can be eliminated. The shaped-charge fuel is applicable for both large power generation as well as for small home heating. |
| Type |
Text |
| Format |
application/pdf |
| Language |
eng |
| Rights |
This material may be protected by copyright. Permission required for use in any form. For further information please contact the American Flame Research Committee. |
| Conversion Specifications |
Original scanned with Canon EOS-1Ds Mark II, 16.7 megapixel digital camera and saved as 400 ppi uncompressed TIFF, 16 bit depth. |
| Scanning Technician |
Cliodhna Davis |
| ARK |
ark:/87278/s669763z |
| Setname |
uu_afrc |
| ID |
4614 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s669763z |