| OCR Text |
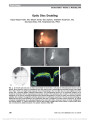
Show Magnetic Resonance Imaging of Optic Neuritis in Patients With Neuromyelitis Optica Versus Multiple Sclerosis Sangeeta Khanna, MD, Aseem Sharma, MD, Julie Huecker, MS, Mae Gordon, PhD, Robert T. Naismith, MD, Gregory P. Van Stavern, MD Background: Patients with neuromyelitis optica (NMO) and multiple sclerosis (MS) both can present with acute optic neuritis (ON), while differing considerably in their prognosis and management. The clinical course, serologic testing results, and brain and spinal cord imaging of these diseases have been well documented. The purpose of this study was to look systematically for any differences in the imaging appearance of the optic nerve in NMO and MS-related ON. Methods: Magnetic resonance imaging (MRI) of brain and orbits obtained within 6 weeks of acute ON in patients with securely diagnosed NMO (n = 6) and MS (n = 11) were retrospectively analyzed by a neuroradiologist masked to the clinical diagnosis. Standardized scoring system was used to assess and analyze the extent and nature of optic pathway involvement. Results: No significant differences were observed in the presence, degree, or the type of signal alteration and contrast enhancement of the affected nerve segments between NMO and MS groups. There was a trend toward more posterior involvement of the optic nerve in the NMO group with chiasmatic enhancement exclusively seen in NMO patients. Conclusion: We found a higher propensity of NMO-related ON to affect more posterior parts of the optic nerve, including chiasm, and have simultaneous bilateral disease. Further study with larger sample sizes is needed. Journal of Neuro-Ophthalmology 2012;32:216-220 doi: 10.1097/WNO.0b013e318254c62d © 2012 by North American Neuro-Ophthalmology Society Optic neuritis (ON) may be the initial presenting fea-ture of both multiple sclerosis (MS) and neuromyelitis optica (NMO). Distinction between the 2 conditions is important, as both diseases differ in pathophysiology, visual and neurologic outcome, and response to treatment (1-3). Early recognition and treatment with immunosuppression can reduce the burden of disease. The clinical spectrum of NMO has expanded considerably with the advent of the NMO immunoglobulin G (IgG) antibody, a sensitive and highly specific test that helps distinguish NMO from MS. The diagnostic criteria for NMO (4) now include a history of ON with history of acute myelitis and 2 of 3 supportive criteria: 1) a contiguous spinal cord MRI lesion extending over at least 3 vertebral segments antibody, 2) a brain MRI that does not meet the criteria for MS, and 3) NMO-IgG seropositivity. Early diagnosis may be critical when making treatment decisions, but there is still debate regarding whether all patients with demyelinating ON need to be screened for NMO. Some recommend testing all patients with ON and aggressive immunosuppression in patients with positive NMO titers (without conclusive evidence of NMO) (5). Others have argued that NMO antibody testing is not war-ranted in every patient because NMO-related isolated ON is rare (5). Clinical parameters such as bilateral onset and poor visual outcomes are often clues to NMO-related ON but are not present in all such patients. A recent study from Brazil demonstrated much higher likelihood of severe residual visual field deficit on automated perimetry after ON related to NMO versus MS (6). Ratchford et al (7) studied a cohort of patients with MS-related ON and NMO-related ON and found significantly greater thinning of retinal nerve fiber layer using optical coherence tomog-raphy (OCT) in NMO patients. Such studies highlight the need for clinical parameters to better guide diagnostic testing. Departments of Ophthalmology and Visual Sciences (SK, JH, MG, GPV); Neuroradiology (AS); and Neurology (RTN, GPV), Washington University School of Medicine, St Louis, Missouri. Research supported by DOVS Core Grant 5 P30 EY02687, Institute for Clinical and Translational Sciences Grant RR023496, Biostat Core Grant U54 RR023496, and an Unrestricted Grant from Research to Prevent Blindness and National Institutes of Health Core Vision Grant P30 EY02687. The authors report no conflicts of interest. Address correspondence to Gregory P. Van Stavern, MD, Departments of Ophthalmology and Visual Sciences and Neurology, Washington University School of Medicine, 660 South Euclid Avenue, St Louis, MO 63110; E-mail: vanstaverng@vision.wustl.edu 216 Khanna et al: J Neuro-Ophthalmol 2012; 32: 216-220 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. MRI plays a critical role in the diagnosis of MS and NMO. Although ON can be diagnosed by clinical features, fat-suppressed orbital MRI shows characteristic findings, including signal change and enhancement of the optic nerve in 95% of the patients, and is often included in the work-up of ON patients (8). Previous studies (8,9) have attempted to use length of enhancement and signal change to predict visual outcome, with variable results. However, no prior research has been done using similar methods to predict neurologic out-come, whether the patient is destined to develop NMO or MS. The goal of this study was to identify any distinctive features of optic nerve imaging with MRI in acute ON in a cohort of securely diagnosed NMO and MS patients. MATERIALS AND METHODS This is a retrospective case study. A chart review consisted of securely diagnosed NMO patients examined and followed by an MS specialist (R.T.N.) between 2006 and 2010. Of the 27 patients with NMO, 6 with NMO-related ON who had neuroimaging done within 6 weeks of onset of visual symptoms were identified and included in the study. Eleven patients with securely diagnosed MS (McDonald criteria) (10) imaged within 6 weeks of onset of ON also were in-cluded. Six weeks was chosen because that is the time frame in which acute findings (such as contrast enhancement of the involved nerve) are most prominent. The MRI of these patients was analyzed by a neuroradiologist (A.S.) masked to the clinical diagnosis. After the protocol was approved by the institutional review board, a retrospective chart review performed to determine the age, sex, race, and the laterality of involvement clinically and to confirm the diagnoses. A standardized scoring system was developed and used to assess and analyze the extent and nature of the optic path-way involvement detected with MRI. MRI was done with a 1.5 Tesla magnet (Siemens AG, Munich, Germany). All patients underwent MRI of the brain and orbits with 5-mm slice thickness. Images included with and without contrast T1 axial, T2 axial, axial fat-suppressed FLAIR, and contrasted T1 coronal. In addition, 10 of the 17 patients had orbital MRI with 3-mm slice thickness. These studies included contrast-enhanced T1 axial and coronal, contrast-enhanced fat-suppressed T1, and fat-suppressed T2. We compared the MRI optic pathway imaging charac-teristics between the 2 groups. The parameters assessed were as follows: 1. Optic nerve enhancement. 2. Degree of optic nerve enhancement: The maximally affected segment of nerve was assessed and graded sub-jectively using level of extraocular muscle enhancement as a reference. 0, none; 1, subtle; 2, definite ,muscles; 3, definite. muscles. 3. Presence of optic nerve thickening. The affected optic nerve segment was compared with contralateral unaf-fected nerve or ipsilateral unaffected segment in bilat-eral cases. 4. Presence of hyperintensity on FLAIR. 5. Degree of hyperintensity on FLAIR: Hyperintensity of signal change was subjectively graded on a scale of 0-3 ranging from no change in signal (0) to marked change in signal (3). 6. Extent of optic nerve involvement: classified as 0, none; 1, up to one-third; 2, one-third to two-third; 3, two-third or greater. 7. Optic nerve head swelling. 8. Segment of nerve most affected (retrobulbar/canalicular/ intracranial). 9. Laterality of involvement. 10. Chiasmal involvement. 11. Optic tract involvement. Fisher exact test (categorical variables) and t tests (contin-uous variables) were used to test for statistical significance. RESULTS Six patients were included in the NMO group and 11 patients in the MS group. The patient characteristics are summarized in Table 1. All patients underwent brain MRI within 6 weeks (range, 4 days to 6 weeks) of an episode of ON. Seven of 11 MS patients and 3 of 6 NMO patients had dedicated orbital MRI in additional brain MRI. Four of 6 NMO patients had unilateral optic nerve involvement, and 2 had bilateral disease. All 11 MS patients had unilat-eral disease. No significant differences were observed between the 2 groups regarding the presence, degree, extent, or the type of signal alteration and contrast enhancement of the affected optic nerve segments. The most important comparison parameters are shown in Table 2. There was a trend toward more posterior optic nerve involvement in the NMO group, with chiasmatic enhancement exclusively seen in NMO-related ON (3 patients, P = 0.0179). Optic nerve head enhancement was not seen in either group. The mean difference in the thickness of the most affected segment compared with unaffected contralateral nerve was higher in MS (mean 0.7182 ± 0.7) versus NMO TABLE 1. Demographic characteristics of NMO and MS patients with ON Characteristics NMO (n = 6) MS (n = 11) Age, mean (SD), y 39 (18.4) 39 (16.7) Female gender, n (%) 5 (83.3) 8 (72.7) Race, n (%) African American 4 (66.7) 4 (36.4) Caucasian 2 (33.3) 7 (63.6) MS, multiple sclerosis; NMO, neuromyelitis optica; ON, optic neuritis. Khanna et al: J Neuro-Ophthalmol 2012; 32: 216-220 217 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. (mean 0.375 ± 0.33), but this was not statistically signifi-cant. The characteristic MRI features of 1 representative MS and 1 NMO patient are shown in Figures 1 and 2, respectively. DISCUSSION In patients with ON caused by NMO and MS, our study showed that there were no distinguishing optic nerve features on MRI. However, bilateral optic nerve and chiasmal enhancement were seen only in our NMO cohort of patients. To our knowledge, there is no prior systematic compar-ison of imaging of acute ON in securely diagnosed NMO versus MS. Prior MRI studies have looked at imaging findings in acute ON but have not systematically included or excluded NMO. Kupersmith et al (8) reviewed MRI findings in 107 patients with acute ON and found abnor-mal optic nerve enhancement in 94% of affected nerves. The percent of abnormal contrast enhancement in our study is similar but slightly less at 80% (NMO patients) to 88.9% (MS patients). One hundred percent of our NMO and 82% our MS patients showed optic nerve hyper-intensity on FLAIR sequences. Although Kupersmith et al found that location and length of optic nerve enhancement are not predictive of visual recovery, a prior report (9) suggested that abnormal signal length .17.5 mm and canalicular location are associated with poor or slow recov-ery from ON even if treated with steroids (9). In the study by Kupersmith et al (8), 17 of 107 patients had probable or definite MS at the time of presentation. It is unclear whether patients were systemically evaluated for NMO. Although NMO patients have poorer visual recovery and more loss of nerve fibers on OCT with each ON episode (2,7), we did not TABLE 2. MRI characteristics of ON in patients with NMO and MS Characteristic NMO (n = 6), % MS (n = 11), % Presence of nerve enhancement* 4 (80.0) 8 (88.9) Presence of nerve thickening 4 (66.7) 7 (63.6) Hyperintensity of nerve on FLAIR 6 (100.0) 9 (81.8) Chiasmal involvement* 3 (60.0) 0 (0) Optic tract involvement 1 (16.7) 0 (0) Optic nerve head swelling 0 0 Most affected segment is retrobulbar 0 (0) 3 (27.0) Most affected segment is canalicular 4 (80.0) 8 (72.7) Most affected segment is intracranial 1 (20.0) 0 Bilateral disease on MRI 2 (33.3) 0 Extent of nerve involvement , one-third 4 (66.7) 5 (45.5) Extent of nerve involvement one-third to two-third 1 (16.7) 2 (18.2) Extent of nerve involvement . two-third 1 (16.7) 2 (18.2) *1 NMO and 2 MS patients had indeterminate call on nerve enhancement. One patient in NMO group had indeterminate call on chiasmal enhancement. The percentage reflects the number positive out of those with determinate call and the percent may be higher than actual number but is more reflective of the true call. Because of a small sample size, the statistical power of comparisons above is low; hence, the P values are not meaningful and have not been included here. MS, multiple sclerosis; NMO, neuromyelitis optica; ON, optic neuritis. FIG. 1. MRI in MS. Contrast-enhanced fat-suppressed T1 MRIs show enhancement of the canalicular and intracranial right optic nerve (arrow) in the axial projection (A) and the intracranial portion (arrowhead) in the coronal view (B). Axial FLAIR image through the brain demonstrates hyperintense foci in the white matter representing demyelination plaques (C). 218 Khanna et al: J Neuro-Ophthalmol 2012; 32: 216-220 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. detect any significant difference in the length of involved segment of optic nerve between the MS and NMO patients. However, a trend toward more posterior location and chias-mal enhancement was noted in our NMO cohort. Chiasmal involvement previously has been reported in NMO (11), and it has also been described in MS based on autopsy and clinical and imaging evidence. Most of these reports were prior to 1991, when NMO had not yet been defined as an entity distinct from MS (12). However, in 2009, Kawasaki et al (13) reported a series of 20 patients with idiopathic chiasmal neuritis and found that 40% developed clinically definite MS, with follow-up ranging from 3 months to 3 years. This series did not exclude NMO patients, and 1 patient developed a myelopathy. In our study, chiasmal enhancement/enlargement was exclu-sively present in the NMO group. We found simultaneous bilateral enhancement of the optic nerves only in our NMO patients. In a study of 427 patients with MS, simultaneous bilateral disease was rare, noted in only 2 patients (0.42%) (14). This suggests that simultaneous bilateral optic nerve enhancement in a monoc-ularly symptomatic patient should warrant careful evalua-tion for NMO. In our study, 1 of the 2 patients with bilateral optic nerve enhancement had clinical findings of unilateral ON. In conclusion, our study suggests that bilateral optic nerve or chiasmal enhancement on MRI might prompt further patient evaluation for NMO. The strength of our study is that we have only included securely diagnosed NMO or MS patients, and MRI studies were reviewed by neuroradiologist who was blinded to the clinical diagnosis. Limitations of our study include its retrospective nature, small patient cohort, and absence of dedicated orbital imaging protocol in all patients. Although studies with larger sample size and standardized imaging are warranted, our findings might serve as a useful guide in clinical decision making when managing patients with acute ON. REFERENCES 1. Merle H, Olindo S, Bonnan M, Donnio A, Richer R, Smadja D, Cabre P. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology. 2007;114:810-815. 2. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999;53:1107-1114. 3. Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: clinical predictors of a relapsing course and survival. Neurology. 2003;60:848-853. 4. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485-1489. 5. Galetta S, Cornblath W. Should most patients with optic neuritis be tested for neuromyelitis optica antibodies and should this affect their treatment? J Neuroophthalmol. 2010;30:376-379. 6. Fernandes DB, Ramos RD, Falcochio C, Apóstolos-Pereira S, Callegaro D, Monteiro ML. Comparison of visual acuity and automated perimetry findings in patients with neuromyelitis optica or multiple sclerosis after single or multiple attacks of optic neuritis. J Neuroophthalmol. 2011 Dec 6 (epub ahead of print). FIG. 2. MRI in NMO. Contrast-enhanced T1 axial (A) and coronal (B) MRIs reveal enhancement of both prechiasmal optic nerves (arrows) and optic chiasm (arrowheads). T2 sagittal spine MRI demonstrates longitudinally extensive myelitis typical of NMO. Khanna et al: J Neuro-Ophthalmol 2012; 32: 216-220 219 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 7. Ratchford JN, Quigg ME, Conger A, Frohman T, Frohman E, Balcer LJ, Calabresi PA, Kerr DA. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009;73:302-308. 8. Kupersmith M, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain. 2002;125:812-822. 9. Dunker S, Wiegand W. Prognostic value of magnetic resonance imaging in monosymptomatic optic neuritis. Ophthalmology. 1996;103:1768-1773. 10. Polman CH, Reingold SC, Banwell B, Clanet M, Cohan JA, Flippi M, Fujihara K, Hardova E, Hutchison M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wolheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions of the "McDonald criteria". Ann Neurol. 2011;69:292-302. 11. Costa RM, Santos AC, Costa LS. An unusual chiasmal visual defect in a patient with neuromyelitis optica: case report. Arq Bras Oftalmol. 2007;70:153-155. 12. Newman NJ, Lessell S, Winterkorn JM. Optic chiasmal neuritis. Neurology. 1991;41:1203-1210. 13. Kawasaki A, Purvin V. Idiopathic chiasmal neuritis: clinical features and prognosis. Arch Ophthalmol. 2009;127:76-81. 14. Burman J, Raininko R, Fagius J. Bilateral and recurrent optic neuritis in multiple sclerosis. Acta Neurol Scand. 2011;123:207-210. 220 Khanna et al: J Neuro-Ophthalmol 2012; 32: 216-220 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |