| OCR Text |
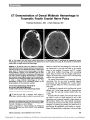
Show Irreversible Optic Neuropathy in Wernicke Encephalopathy and Leber Hereditary Optic Neuropathy John-Michael Li, MD, Janet C. Rucker, MD Abstract: A 52-year-old woman with alcohol abuse presented with recent worsening of vision, imbalance, and confusion. Examination revealed counting fingers acuity in both eyes with central scotomas, color vision loss, horizontal nystagmus, and gait ataxia. Thiamine was initiated as treatment for a presumptive diagnosis of Wernicke encephalopathy (WE). Brain MRI revealed high T2 signal in the dorsal midbrain and thalami character-istic of WE. The lack of optic disc edema, usually present in patients with WE who have severe optic neuropathy, and lack of visual loss reversibility with thiamine treatment, led to the suspicion of coexisting Leber hereditary optic neuropathy (LHON), which was later confirmed when testing revealed the 14484 mitochon-drial DNA mutation. Over the ensuing months, vision did not recover despite improvement of other neurologic findings. Irreversible optic neuropathy in WE should prompt consideration of a coexisting mitochondrial disorder such as LHON. Journal of Neuro-Ophthalmology 2010;30:49-53 doi: 10.1097/WNO.0b013e3181ce80c6 2010 by North American Neuro-Ophthalmology Society Visual loss in the setting of Wernicke encephalopathy (WE) has been reported rarely in addition to the classically recognized triad of ataxia, ophthalmoplegia, and confusion (Table 1) (1-13). Visual loss is typically severe and bilateral with visual acuity worse than 20/200 accompanied by central and cecocentral scotomas on formal visual field testing. Ophthalmoscopic examination most often reveals bilateral disc edema, but patients with normal optic discs at the time of vision loss are reported (4,9). Patients with WE normally have rapid improvement of symptoms, including visual loss, with thiamine supplementation (1-13). We report a patient with the classic triad of WE and bilateral retrobulbar optic neuropathies who, despite thiamine supplementation, had minimal improvement of visual loss. Further workup revealed the 14484 mutation of Leber hereditary optic neuropathy (LHON). We propose that there may be a contribution of LHON to irreversible visual loss in WE. CASE REPORT A 52-year-old woman with a history of long-standing severe alcohol abuse and a 35-year history of cigarette use presented with a 2-week history of painless bilateral visual loss of sudden onset preceded by a vague 2-month history of mildly blurred vision. She also complained of imbalance. Her family had noted mild recent confusion. Family history was significant only for glaucoma in her father. Visual acuity was counting fingers bilaterally at distance and 20/800 at near. She could not see the Ishihara control plate with either eye. On dilated ophthalmoscopic examina-tion, there was subtle temporal optic nerve pallor bilaterally, considered possibly physiologic. Goldmann visual fields revealed bilateral cecocentral scotomas. Saccades, smooth pursuit, optokinetic nystagmus, and vestibulo-ocular responses were normal. Intermittent low-amplitude ‘‘shim-mering'' horizontal nystagmus was detectable in primary position and became more prominent on upward gaze. A formal mental status examination was not performed, but the patient had slowed cognitive responses, was disinhi-bited, and recalled only 1 of 3 objects at 5 minutes. She had mild bilateral upper extremity tremulousness and marked difficulty with tandem gait. There were no signs of a peripheral neuropathy. The optic neuropathy was attributed to a metabolic/toxic process. The combination of confusion, nystagmus, and gait ataxia in a severe alcoholic was suggestive of WE. Thiamine supplementation was immediately initiated and discontin-uation of alcohol and tobacco was recommended. MRI of the brain and orbits showed increased T2 signal in the dorsal midbrain and bilateral periventricular thalami, Department of Neurological Sciences (J-ML), Rush University Medical Center, Chicago, Illinois; and Departments of Neurology and Ophthalmology (JCR), Mount Sinai Medical Center, New York, New York. Address correspondence to Janet C. Rucker, MD, Mount Sinai Medical Center, One Gustave L. Levy Place, Box 1052, New York, NY 10029; E-mail: janet.rucker@mssm.edu Li and Rucker: J Neuro-Ophthalmol 2010; 30: 49-53 49 Original Contribution supportive of the clinical suspicion of WE (Fig. 1). The vitamin B12 level was normal, and results for methanol and alcohol, a rapid plasmin reagin (RPR) test, and a fluorescent treponemal antibody absorption (FTA-ABS) test were negative. The thiamine level was 0.3 mg/dL, at the lower limit of normal for our laboratory. Testing for the 3 common mitochondrial mutations for LHON was positive for the 14484 mutation. The patient's final diagnoses were LHON and WE of simultaneous onset. At a 4-month follow-up examination, ataxia and nystagmus had diminished. There was no change in visual function. Repeat brain MRI revealed lessening of the previously noted T2 signal abnormalities. DISCUSSION Our patient had features that supported a diagnosis of WE-a long history of severe alcohol abuse, recent mild confusion reported by her family, ataxia, and nystagmus. Supportive evidence included a low-normal thiamine level and the MRI T2 and FLAIR hyperintensities in the dorsal midbrain and periventricular thalami. Supplementation of thiamine improved the nystagmus and ataxia but not her vision. The lack of visual improvement is atypical for the visual loss previously reported with WE, but characteristic for LHON. The severely decreased acuity and central and cecocentral scotomas suggested a metabolic/toxic process, such as that caused by mitochondrial dysfunction in drug toxicities and LHON. Although our patient lacked the classic acute optic disc features of peripapillary telangiectatic microangiopathy and retinal nerve fiber layer elevation and hyperemia typically seen with LHON, the absence of such features does not exclude LHON as the cause of visual loss. Importantly, she differed from previously reported patients with visual loss in WE, who have typically manifested bilateral disc edema (Table 1) (4,9). The few patients with normal optic nerve appearance at presentation have had rapid improve-ment of visual deficits after thiamine supplementation (9). The mechanism of disc swelling in WE was initially considered to be papilledema from raised intracranial pressure (4). However, cerebrospinal fluid pressure has been normal. Moreover, visual loss has, in most patients, been more severe than that associated with acute or subacute papilledema. Inflammation was therefore later proposed as the mechanism of optic neuropathy (8). The patients lacking optic disc edema suggest yet another mechanism: mitochondrial dysfunction. Thiamine deficiency has numerous effects at the mito-chondrial level, as phosphorylated thiamine is a cofactor for several mitochondrial enzymes including a-ketogluta-rate dehydrogenase, pyruvate dehydrogenase, and trans-ketolase. In thiamine-deficient animal models, malfunction of a-ketoglutarate dehydrogenase is responsible for tissue damage via failure of the tricarboxylic acid (TCA) cycle and resultant impaired cellular production of ATP (14,15). Pathologic studies have shown that thiamine deficiency has a selective effect on neuronal populations in the thalamus and brainstem, corresponding to the location of neuroimaging abnormalities in human WE (15,16). The mechanisms of cellular death appear to be related to secondary formation of a focal lactic acidosis, N-methyl-d-aspartate receptor-mediated excitotoxicity, and failure of energy production. In humans, thiamine deficiency can have manifestations beyond WE. Patients from a family with the A3243G mito-chondrial mutation, mitochondrial myopathy, and familial FIG. 1. Axial FLAIR MRI reveals increased signal in the dorsal midbrain (A) and periventricular thalamus (B), findings typical of Wernicke encephalopathy. Original Contribution 50 Li and Rucker: J Neuro-Ophthalmol 2010; 30: 49-53 TABLE 1. Vision loss in Wernicke encephalopathy (all visual acuities bilateral unless noted otherwise, all acuities converted to Snellen) Reference Patient Age/Sex (Setting) Symptoms Signs Visual Acuity Optic Disc Edema Tests Outcome (time after treatment initiated) Mumford13 24 W (HG) nausea, decreased focusing ability mental status changes, ataxia, nystagmus (H,V), coma after intravenous glucose administration N/A Yes CT NL CSF - OP and protein NL Acuity 20/20; Fields and optic disc NL Timmings10 47 W (Alcoholism) diplopia, sudden vision loss mental status changes, nonreactive pupils, horizontal gaze paresis, nystagmus (V), ataxia No light perception N/A CT ventricular dilation Count fingers both eyes (4 hours); 20/125 Right eye, 20/80 Left eye (2 weeks); Fields NL (21 days); 20/20 (27 days) Suzuki3 22 M (TPN) sudden vision loss, oscillopsia nystagmus (V) bilateral sixth cranial nerve palsies , 20/200 Yes MRI consistent with WE Full recovery Tesfaye5 21 W (HG) vision loss to blindness over 10 days confusion, ‘‘restricted gaze'', nystagmus (H, V) Unable to count fingers at 1 meter Yes MRI NL 20/20 Right eye, 20/32 Left eye (24 hours); NL (3 weeks) Halavaara1 20 W (Chronic vomiting) N/A gaze palsies, nystagmus (H,V) ‘‘reduced acuity'' Yes MRI consistent with WE Marked improvement Gokce6 47 W (TPN) confusion bilateral sixth cranial nerve palsies, nystagmus (H), sluggish pupils, progressed to coma ‘‘Profound vision loss'' Yes MRI NL CSF protein elevated, OP N/A 20/40 Right eye, 20/63 Left eye (1 month); 20/32 Right eye, 20/50 Left eye VEP delayed right eye NL Left eye walking a few steps (1 year) Ferdinands12 22 W (HG) vision loss, diplopia, progressed to coma after given glucose ataxia, right gaze paresis, confusion 20/125 Right eye 20/63 Left eye Yes MRI consistent with WE; CSF NL; OP N/A Normal vision (3 days) (continued on next page) Original Contribution Li and Rucker: J Neuro-Ophthalmol 2010; 30: 49-53 51 TABLE 1. (continued) Reference Patient Age/Sex (Setting) Symptoms Signs Visual Acuity Optic Disc Edema Tests Outcome (time after treatment initiated) Kulkarni8 24 W (Gastric bypass) vision loss, imbalance ataxia, nystagmus (H, GEN) Light perception, central field loss Yes MRI consistent with WE; CSF NL; OP NL 20/30 Right eye, 20/20 Left eye, visual fields NL (24 hours) Cooke7 11 W (11 weeks of vomiting) vision loss, ‘‘central haze'' horizontal gaze paresis, nystagmus (upbeat) 20/200 Right eye 20/80 Left eye Yes MRI consistent with WE; CSF NL; OP N/A 20/40 Right eye, 20/80 Left eye (24 hours); 20/40, Ishihara color NL, optic discs NL (2 weeks) Wilson11 17 W (HG) confusion, blurred vision right gaze paresis, nystagmus (GEN), ataxia N/A Yes MRI consistent with WE; CSF NL; OP NL Improved over days Surges9 37 M (Alcoholism) sudden onset blindness mental status changes, bilateral sixth cranial nerve palsies, nystagmus (downbeat), dilated pupils, ataxia No light perception No MRI NL NL (12 hours) Longmuir2 34 W (Gastric bypass) vision loss, diplopia horizontal gaze paresis, nystagmus (GEN), ataxia 20/200 Right eye, 20/70 Left eye Yes MRI NL 20/20, Goldmann visual fields NL (14 hours) Current 52 W (Alcoholism) vision loss, confusion, imbalance nystagmus (H), ataxia Counting fingers, central scotomas both eyes No MRI consistent with WE No recovery DBN, downbeat nystagmus; GEN, gaze-evoked nystagmus; H, horizontal; HG, hyperemesis gravidarum; M, man; N/A, not available; NL, normal; OP, opening pressure; TPN, total parenteral nutrition; V, vertical; VEP, visual evoked potential; W, woman; WE, Wernicke encephalopathy. Original Contribution 52 Li and Rucker: J Neuro-Ophthalmol 2010; 30: 49-53 thiamine deficiency have shown improved muscle strength and decreased lower extremity edema with thiamine supplementation (17). Thiamine-responsive peripheral neu-ropathies and optic neuropathies have also been documented in patients later found to have pyruvate dehydrogenase deficiencies (18). It is speculated that in patients with mitochondrial abnormalities, tolerance to decreased thiamine may be lower and thus higher levels of thiamine may be required to compensate for impaired cellular energy pro-duction. In the setting of malnutrition or thiamine malabsorption, these individuals may be more likely to develop WE. Such a phenomenon may have occurred in our patient, given the simultaneous development of visual loss and WE in the setting of a low-normal thiamine level. Although our patient displayed the classic clinical triad and the imaging findings of WE, we cannot completely exclude the possibility that these features were a manifesta-tion of her mitochondrial mutation alone. Brain lesions on MRI are present in a small percentage of patients with LHON. However, these are most commonly reported in 1 of 2 clinical settings: 1) in patients with a relapsing, remitting illness indistinguishable from multiple sclerosis (MS); and 2) in patients who are neurologically normal except for the visual loss associated with LHON (19). The brain lesions are also characteristic of MS, with multiple T2 hyperintensities in periventricular and subcortical cerebral white matter, brainstem, and cerebellum. Such imaging findings were not seen in our patient. There is a report of 3 patients with the common LHON mutations who developed an illness resembling Leigh disease with imaging features similar to those of our patient (20). There is overlap between the imaging abnormalities in those reported patients and in WE, with prominent midbrain and periventricular thalamic lesions in both. In contrast to our patient's monophasic course of thiamine-responsive ataxia and nystagmus with simultaneous vision loss, none of those patients had visual loss temporally related to an illness with features of Leigh disease. Moreover, all 3 patients had a relapsing or progressive neurologic illness over many years. REFERENCES 1. Halavaara J, Brander A, Lyytinen J, et al. Wernicke's encephalopathy: is diffusion-weighted MRI useful? Neuroradiology 2003;45:519-23. 2. Longmuir R, Lee AG, Rouleau J. Visual loss due to Wernicke syndrome following gastric bypass. Semin Ophthalmol 2007;22:13-9. 3. Suzuki S, Kumanomido T, Nagata E, et al. Optic neuropathy from thiamine deficiency. Intern Med 1997;36:532. 4. Bleggi-Torres LF, de Medeiros BC, Ogasawara VS, et al. Iatrogenic Wernicke's encephalopathy in allogeneic bone marrow transplantation: a study of eight cases. Bone Marrow Transplant 1997;20:391-5. 5. Tesfaye S, Achari V, Yang YC, et al. Pregnant, vomiting, and going blind. Lancet 1998:352:1594. 6. Gokce M, Bulbuloglu E, Tuncel D, et al. Nonalcoholic Wernicke's encephalopathy with prominent astasia and optic neuropathy. Med Princ Pract 2005;14:438-40. 7. Cooke CA, Hicks E, Page AB, et al. An atypical presentation of Wernicke's encephalopathy in an 11-year-old child. Eye 2006;20:1418-20. 8. Kulkarni S, Lee AG, Holstein SA, et al. You are what you eat. Surv Ophthalmol 2005;50:389-93. 9. Surges R, Beck S, Niesen WD, et al. Sudden bilateral blindness in Wernicke's encephalopathy: case report and review of the literature. J Neurol Sci 2007;260:261-4. 10. Timmings PL, Carroll GJ, Donaldson IM. Wernicke's encephalopathy presenting with blindness. N Z Med J 1993; 106:159-60. 11. Wilson RK, Kuncl RW, Corse AM. Wernicke's encephalopathy: beyond alcoholism. Nat Clin Pract Neurol 2006;2:54-8. 12. Ferdinands MD, Seneviratne J, White O. Visual deterioration in hyperemesis gravidarum. Med J Aust 2005;182:585-96. 13. Mumford CJ. Papilloedema delaying diagnosis of Wernicke's encephalopathy in a comatose patient. Postgrad Med J 1989;65:371-3. 14. Desjardins P, Butterworth RF. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke's encephalopathy. Mol Neurobiol 2005;31:17-25. 15. Pannunzio P, Hazell AS, Pannunzio M, et al. Thiamine deficiency results in metabolic acidosis and energy failure in cerebellar granule cells: an in vitro model for the study of cell death mechanisms in Wernicke's encephalopathy. J Neurosci Res 2000;62:286-92. 16. Shi Q, Karuppagounder SS, Xu H, et al. Responses of the mitochondrial -ketoglutarate dehydrogenase complex to thiamine deficiency may contribute to regional selective vulnerability. Neurochem Int 2007;50:921-3. 17. Sato Y, Nakagawa M, Higuchi I, et al. Mitochondrial myopathy and familial thiamine deficiency. Muscle Nerve 2000;23:1069-75. 18. Sedel F, Challe G, Mayer JM, et al. Thiamine responsive pyruvate dehydrogenase deficiency in an adult with peripheral neuropathy and optic neuropathy. J Neurol Neurosurg Psychiatry 2008;79:846-57. 19. Kuker W, Weir A, Quaghebeur G, et al. White matter changes in Leber's hereditary optic neuropathy: MRI findings. Eur J Neurol 2007;14:591-3. 20. Funalot B, Reynier P, Vighetto A, et al. Leigh-like encephalopathy complicating Leber's hereditary optic neuropathy. Ann Neurol 2002;52:374-7. Original Contribution Li and Rucker: J Neuro-Ophthalmol 2010; 30: 49-53 53 |