| OCR Text |
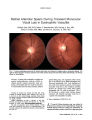
Show ORIGINAL CONTRIBUTION Stereotactic Radiosurgery in Two Cases of Presumed Fourth Cranial Nerve Schwannoma Evis Petrela, MD, Charles J. Hodge, MD, Seung S. Hahn, MD, Chung T. Chung, MD, and Luis J. Mejico, MD Abstract: A 47-year-old woman and a 45-year-old man with gradually progressive fourth cranial nerve palsy underwent stereotactic radiosurgery for pre-sumed fourth cranial nerve schwannomas with the gamma knife at a marginal tumor dose of 14 and 13 Gy, respectively. In one patient, the ocular misalignment disappeared; in the other patient, it stabilized. MRI showed shrinkage of the tumors. These patients represent the second and third reported cases of presumed fourth cranial nerve schwannoma treated with radiosurgery and the first cases with substantial follow-up information. (J Neuro-Ophthalmol 2009;29:54-57) ntracranial schwannomas have been reported to occur in association with neurofibromatosis and less commonly without this condition (1-3). Usually they arise from sensory nerves but they have also been reported in a number of mixed and purely motor cranial nerves. Fourth cranial nerve schwannomas may present clinically by causing dysfunction of the nerve from which they arise by causing dysfunction of neighboring structures (1-3). Clinical and radiological observation has been suggested for small isolated fourth cranial nerve schwannomas measuring less than 5 mm in size (1), whereas surgical intervention is recommended for larger lesions with mass effect on neighboring structures (2-5). We report the clinical outcome at 2 years of patients with gradually progressive fourth cranial nerve palsy caused by presumed schwannomas arising within that nerve and treated with stereotactic radiosurgery. I From the Departments of Ophthalmology (EP, LJM), Neurosurgery (CJH), Radiation Oncology (SSH, CTC), and Neurology (LJM), SUNY Upstate Medical University, Syracuse, New York. This work was supported by unrestricted grants from Research to Prevent Blindness, Inc., New York City, NY, and Lions District 20-Y1. Address correspondence to Luis J. Mejico, MD, Department of Neurology, 90 Presidential Plaza, Syracuse, NY 13202; E-mail: mejicol@ upstate.edu CASE REPORTS Case 1 A 47-year-old woman presented with new binocular diplopia of 6 weeks' duration. There had been no head trauma, and the patient lacked conventional risk factors for arteriosclerosis. There were no other ocular or neu-rologic symptoms, and there was no family history of neurofibromatosis. The ophthalmologic examination was normal except for left hypertropia of 4 prism-diopters in primary gaze position that worsened to 6 prism-diopters on right gaze and on left head tilt. Skin examination did not reveal the stigmata of neurofibromatosis. The baseline complete blood count, cholesterol levels, fasting glucose levels, thyroid tests, and acetylcho-line receptor antibodies showed no abnormalities. Brain MRI (Fig. 1) demonstrated a left enhancing extra-axial lesion adjacent to the midbrain just below the tentorial notch, measuring 7 mm in the largest diameter. Cerebral angiography revealed no evidence of aneurysm or vascular malformation. A presumptive diagnosis of left fourth cranial nerve palsy caused by schwannoma was made. No intervention occurred, but at 3 months the left hypertropia in primary gaze had worsened to 6 prism-diopters and at 6 months to 10 prism-diopters, so the patient underwent stereotactic radiosurgery 6 months after the onset of symptoms. The procedure was performed using the Leksell gamma knife (Elektra Instruments, Norcross, GA). An 8-mm collimator time single shot was used to deliver a marginal tumor dose of 14 Gy to the 70% isodose line. The tumor volume was measured to be 0.168 mL. A few months after treatment, the patient reported resolution of diplopia. She remained asymptomatic after 2 years. Examination then disclosed normal ocular align-ment in all gaze positions. MRI at 2 years after treatment showed a decrease in the size of the lesion (Fig. 1). Case 2 A 45-year-old man presented with new diplopia of 8 weeks' duration. There had been no head trauma and the 54 J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 Fourth Cranial Nerve Schwannoma J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 FIG. 1. Case 1. Brain MRI performed before (A-C) and 2 years after (D-F) stereotactic radiosurgery for a presumed left fourth cranial nerve schwannoma. There is an extra-axial lesion (arrows) adjacent to the midbrain below the tentorial notch measuring 7 mm in the largest diameter. It is isointense to brain parenchyma on precontrast T1 (A) and T2 (B) images and enhances homogeneously on the postcontrast T1 (C) image. Two years after treatment, there is a decrease in the size of the lesion on corresponding MRI sequences (D-F). patient lacked conventional risk factors for arteriosclerosis. There were no other ocular or neurological symptoms, and there was no family history of neurofibromatosis. Ophthalmic examination was normal except for a right hypertropia of 4 prism-diopters in primary gaze position that worsened to 18 prism-diopters in left gaze and 9 prism-diopters on right head tilt. One month earlier, the referring ophthalmologist had documented a right hyper-tropia of 2 prism-diopters in primary gaze. Skin exami-nation did not reveal the stigmata of neurofibromatosis. The baseline complete blood count, cholesterol levels, fasting glucose levels, thyroid tests, and acetylcho-line receptor antibodies showed no abnormalities. Brain MRI demonstrated a 3-mm enhancing lesion in the right side of the midbrain (Fig. 2). A presumptive diagnosis of right fourth nerve palsy caused by schwannoma was made. Initial management consisted of clinical observation, but the patient's right hypertropia in primary gaze had increased to 6 prism-diopters at the 3-month follow-up visit and to 8 prism-diopters at the 6-month follow-up visit. 55 J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 Petrela et al FIG. 2. Case 2. Brain MRI performed before (A-C) and 2 years after (D-F) stereotactic radiosurgery for a presumed right fourth cranial nerve schwannoma. There is an extra-axial lesion (arrows) adjacent to the midbrain measuring 3 mm in its largest diameter. It is isointense on precontrast T1 (A) and T2 (B) images and homogenously enhances on the postcontrast T1 (C) image. Two years after treatment, there is a slight decrease in the size of the lesion on corresponding MRI sequences (D-F). Therefore, at 6 months after the onset of symptoms, the patient underwent stereotactic radiosurgery using the Leksell gamma knife. A 4-mm collimator time single shot was used to deliver a marginal tumor dose of 13 Gy at the 68% isodose line. The tumor volume was measured to be 0.039 mL. At the 2-year follow-up visit, the ocular alignment had not changed. MRI at that time showed a decrease in the size of the lesion (Fig. 2). DISCUSSION Our two patients presented with worsening binocular diplopia and their ocular alignment measurements were consistent with gradually progressive fourth cranial nerve palsy. In each case, MRI demonstrated an enhancing lesion along the path of the fourth cranial nerve at the midbrain level. Common intrinsic lesions of the fourth cranial nerve include schwannomas, neurofibromas, neurilemomas, hemangiomas, and their malignant counterparts (1,6). Rarely CSF spread of metastatic disease and lymphomas can appear as focal cranial nerve masses (7). The ultimate diagnosis of a tumor in this location requires histologic evaluation, but biopsy is likely to cause permanent dysfunction of the nerve. Improvement in neuroimaging techniques has offered advantage in the diagnosis of these tumors. Mulkens et al (8) reviewed the MRI characteristics of 84 acoustic schwannomas, including 52 with pathologic diagnosis, and found that precontrast isointensity of the lesion to brain parenchyma and postcontrast intense homogeneous enhancement were characteristic of schwannoma. Cranial nerve schwannomas occur rarely in the absence of neurofibromatosis, as was the case in our two patients. Accounting for approximately 8% of intracranial neoplasms (1), they most commonly arise from sensory (vestibular and trigeminal) nerves. (6). Schwannomas of motor nerves and, more specifically of the fourth cranial nerve, are extremely rare. There are only 36 reported cases (4), 27 confirmed by biopsy (2). Their natural course is therefore not well known. Left untreated, they usually enlarge gradually (3) and may rarely undergo intrinsic bleeding that causes rapid worsening of symptoms (4). On occasion, they may remain stationary (1). Feinberg et al (1) 56 q 2009 Lippincott Williams & Wilkins Fourth Cranial Nerve Schwannoma J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 reported 6 cases of schwannomas that measured less than 5 mm in greatest diameter in patients who were followed over a period ranging from 11 to 26 months. Five tumors did not demonstrate progression, either clinically or radiologically. These authors suggested that initial obser-vation with serial imaging is the appropriate management for small, isolated presumed schwannomas of the fourth cranial nerve that remain clinically stable. Surgical resection via retrosigmoid (2), subtemporal, and lateral suboccipital (3) approaches has been undertaken when fourth cranial nerve schwannomas measure between 10 and 45 mm in largest diameter and exert mass effect on neighboring tissues causing neurologic deficits such as hemiparesis, cerebellar ataxia, sensory disturbance, head-aches, dysarthria, or vertigo. Unfortunately, morbidity associated with surgical resection can be substantial. All patients who underwent surgery developed permanent complete fourth cranial nerve palsy postoperatively, leading Ture et al. (3) to conclude that it is very difficult to remove these tumors without compromising function. Thus, in our two patients with small tumors who had gradual worsening of their diplopia and strabismus over a 6-month observation period, surgical excision was not a good option given the lack of associated neurologic symptoms and the high reported morbidity. Therefore, the decision was made to treat both with stereotactic radiosurgery. Patients with intracranial schwannomas respond particularly well to stereotactic radiosurgery owing to the noninfiltrative nature of the tumor and the relatively high resistance of most cranial nerves to radiation toxicity (9-11). Stereotactic radiosurgery is preferred over frac-tionated therapy because of its convenience (treatment is completed in 1 day) and the fact that it provides precise delivery of radiation to small targets with rapid dose fall-off so as to spare adjacent structures (12). Stereotactic radiosurgery has been performed suc-cessfully in patients with small to moderate size vestibular (10) and nonvestibular schwannomas without symptomatic mass effect (5) to minimize surgical excision-related morbidity of such benign tumors. Most of the data come from treatment of these relatively common tumors, for which stereotactic radiosurgery offers a high control rate but continues to pose a risk for cranial nerve damage. In a recently published series, Chopra et al (11) reported 98.3% tumor control rate at the 5- to 10-year-follow-up in 216 vestibular schwannomas treated with marginal tumor doses of 12-13 Gy. However, these authors found that progressive hearing loss may continue in the long term. Pollock et al (5) reported 23 patients with small to medium size nonvestibular schwannomas treated with stereotactic radiosurgery and concluded that this treatment offers less morbidity and very low tumor recurrence rate. In their series they included only one patient with a fourth cranial nerve schwannoma and provided no information about clinical outcome for that patient. In our patients, previous radiation dose regimens shown to be safe and effective for treating acoustic schwannomas seemed a reasonable choice (11). Neither patient had complications from treatment. The first patient had complete resolution of symptoms and remained orthophoric at the 2-year follow-up, whereas our second patient had immediate and prolonged stabilization of diplopia at a level well controlled with prism glasses. Posttreatment MRIs demonstrated a decrease in size of the lesions in both patients and repeat MRIs at a 2-year follow-up have not demonstrated regrowth. Our study is limited by the fact that we are reporting only two cases. Further studies and longer follow-up are necessary to demonstrate the short- and long-term efficacy of stereotactic radiosurgery as the primary treatment for small to medium size presumed fourth cranial nerve schwannomas with worsening symptomatology. REFERENCES 1. Feinberg AS, Newman N. Schwannoma in patients with isolated unilateral trochlear nerve palsy. Am J Ophthalmol 1999:183-8. 2. Gerganov V, Samii A, Koerbel A, et al. Cystic trochlear nerve schwannoma: case report. Surg Neurol 2007;68:221-5. 3. Ture U, Ozduman K, Elmaci I, et al. Infratentorial lateral supracerebellar approach for trochlear nerve schwannoma. J Clin Neurosci 2002;9:595-8. 4. Ohba S, Miwa T, Kawase T. Trochlear nerve schwannoma with intratumoral hemorrhage: case report. Neurosurgery 2006; 58:E791. 5. Pollock BE, Foote RL, Stafford SL. Stereotactic radiosurgery: the preferred management for patients with nonvestibular schwannomas. Int J Radiat Oncol 2002;52:1002-7. 6. Russell DS, Rubinstein LJ. Pathology of Tumors of the Nervous System. 5th ed. Baltimore: Williams & Wilkins; 1989:533-89. 7. Malde R, Laskar S, Muckaden MA, et al. Primary, multifocal extranodal lymphoma presenting with multiple cranial nerve palsies: an unusual presentation of an uncommon entity. Leuk Lymphoma 2004;45:389-91. 8. Mulkens TH, Parizel PM, Martin JJ, et al. Acoustic schwannoma: MR findings in 84 tumors. AJR Am J Roentgenol 1993;160:395-8. 9. Franzin N, Vimercali A, Medone M, et al. Neuroophthalmological evaluation of gamma knife surgery for cavernous sinus meningiomas. Neurosurg Focus 2007;23:E10. 10. Kondziolka D, Nathoo N, Flickinger JC, et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery 2003;53: 815-22. 11. Chopra R, Kondziolka D, Niranjan A, et al. Long term follow up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2007;68:845-51. 12. Mack A, Scheib S, Major J, et al. Precision dosimetry for narrow photon beams used in radiosurgery-determination of gamma knife output factors. Med Phys 2002;29:2080-9. 57 [VBneurofibromatosistwo] |