| OCR Text |
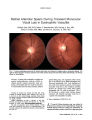
Show ORIGINAL CONTRIBUTION Optic Neuropathy Caused by Naso-Orbital Mass in Chronic Intranasal Cocaine Abuse Christopher C. Shen, MD, Amanda L. Silver, MD, Thomas J. O'Donnell, MD, James C. Fleming, MD, and Zeynel A. Karcioglu, MD We describe a patient in whom chronic intranasal cocaine use produced a bone-destructive, fibro-inflammatory, naso-orbital lesion that damaged the optic nerve and masqueraded histopathologically as a soft tissue neoplasm. The histopathologic findings of cocaine abuse may mimic either a neoplasm or necrotizing vasculitis and could prove especially confusing if a history of cocaine abuse is not known. CASE REPORT A 48-year-old African-American woman presented to the emergency room with a history of slowly progressive proptosis and loss of vision in her right eye with inter-mittent epistaxis over the previous year. She had had uterine fibromyomas and anemia. She admitted to chronic intra-nasal cocaine abuse and heavy cigarette smoking. Visual acuity was count fingers at 1 foot in the right eye and 20/30 in the left eye, with a right afferent pupillary defect. Applanation intraocular pressures were normal. She had 5 mm of right axial proptosis. Extraocular movements were limited in the right eye in all directions. The con-junctiva of the right eye was chemotic and hyperemic. Ophthalmoscopy disclosed marked optic disc pallor in the right eye. Head CT revealed an absent nasal septum accompa-nied by bony erosion of the ethmoid, sphenoid, and maxillary sinuses and an infiltrating mass extending into the right inferomedial orbit (Fig. 1). Perinuclear antineu-trophilic cytoplasmic antibodies (P-ANCA), cytoplasmic antineutrophilic cytoplasmic antibodies (C-ANCA), eryth-rocyte sedimentation rate (ESR), homocysteine, rapid plasma reagin (RPR), and antinuclear antibodies (ANA), were within normal limits. Endoscopic exploration disclosed a large crust-filled nasal cavity with friable mucosa and herniation of the right orbital contents into the nose. Biopsy of the septum, right nasal wall, and periorbita revealed a nonspecific necrotizing inflammatory reaction and vasculitis in the nasal and sinus mucosa composed of polymorphonuclear cells, eosinophils, lymphocytes, and plasma cells (Fig. 2). Gram, periodic acid-Schiff (PAS), and Giemsa stains failed to Abstract: A 48-year-old woman with a history of chronic intranasal cocaine abuse presented with unilateral proptosis associated with severe visual loss from optic neuropathy in the right eye. Imaging showed extensive bone and soft tissue destruction in the paranasal region and an orbital mass. Initial biopsies suggested a low-grade neoplasm. The cor-rect diagnosis was established only on repeat biopsy, which revealed marked pleomorphism and non-specific chronic inflammation with irregular collagen bundles containing thick-walled blood vessels. This case emphasizes that intranasal cocaine abuse may clinically, radiographically, and histopathologically mimic a neoplasm or a necrotizing vasculitis. (J Neuro-Ophthalmol 2009;29:50-53) Cocaine is a naturally occurring alkaloid of the coca plant Erythroxylon coca. Its pharmacologic effects are due to stimulation of the central and sympathetic nervous systems and augmentation of the normal effects of the neurotransmitter norepinephrine. It produces anesthesia by inhibiting excitation of nerve endings or by blocking conduction in peripheral nerves by reversibly binding to and inactivating sodium channels (1,2). Chronic intranasal cocaine abuse is known to cause local vasoconstriction and ischemic necrotizing inflamma-tion of soft tissues, cartilage, and bones of the paranasal sinuses and nasolacrimal drainage system (3,4). Optic neuro-pathy, proptosis, ophthalmoplegia, corneal lesions, and transient monocular blindness have also been reported (4-6). Hamilton Eye Institute (CCS, TJO, JCF, ZAK), University of Tennessee, Health Sciences Center, Memphis, Tennessee; and Department of Otolaryngology (ALS), Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts. Address correspondence to Thomas J. O'Donnell, MD, Assistant Professor of Ophthalmology Neuro-Ophthalmology, Hamilton Eye Institute, University of Tennessee, Health Sciences Center, 930 Madison Ave., Suite 470, Room 485, Memphis, TN 38163; E-mail todonnel@ utmem.edu. This work was supported in part by Research to Prevent Blindness (ZAK, TJO, JCF) and St. Giles Foundation of New York (ZAK). 50 J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 Cocaine Abuse J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 FIG. 1. Axial (A), coronal (B), and sagittal (C) CT performed at presentation show forward and lateral displacement of the right eye and a large nasosinusoidal defect communicating with the right orbit (arrows). A poorly delineated inflammatory mass is best seen on the coronal and sagittal views (asterisk). reveal any causative organisms. Nasal cultures revealed multiple organisms, including Proteus mirabilis, Staphy-lococcus aureus, and group A -streptococcus. She received a course of intravenous vancomycin and ceftriaxone. Because of worsening proptosis, she underwent an inferior orbitotomy and orbital incisional biopsy 2 months later. This sample disclosed necrotizing inflammation and extensive fibrosis. Because of the active nature of the fibrous proliferation, a low-grade fibrous tumor could not be excluded. Because of persistent proptosis, she underwent an orbital decompression procedure 7 weeks later. Tissue samples from this third procedure showed nonspecific chronic inflammation and active fibrosis. Diffuse, irregular collagen bundles containing thick-walled blood vessels with focal chronic inflammatory cell deposits were present. In some areas fibroblastic proliferation was exuberant. Although there was marked pleomorphism, no mitotic figures were seen (Fig. 3). No amyloid was detected with Congo red stain. Pathologists were now satisfied that this was not a neoplasm or a primary vasculitis. DISCUSSION This case illustrates that chronic cocaine abuse may produce confusing histopathologic abnormalities and that several biopsies may be needed to clarify the diagnosis. In otorhinolaryngologic and oculoplastic procedures, cocaine may be used topically in very short duration for its vasoconstrictive and local anesthetic properties without untoward consequences (7). When cocaine is used chronically, however, these actions become detrimental. Vasoconstriction induces mucosal and submucosal tissue ischemia and leads to varying degrees of destruction of soft and bony tissues (8-11). With long-standing intranasal use, cocaine causes progressive destruction of the mid-facial structures, including the hard and soft palate, nasal septum, nasal turbinates, nasal ala, ethmoid sinuses, and adjacent tissues. Patients may ultimately develop nasal collapse, including saddle-nose deformity and oronasal fistula (8-13). The pathophysiology of intranasal tissue loss includes secondary infection, impaired mucociliary transport, de-creased humoral and cell-mediated immunity, and chemical irritation from adulterants put into ‘‘cut'' cocaine (3,14). Optic neuropathy in chronic cocaine abusers is thought to be due to ischemic necrosis of the soft tissues in close proximity to the optic nerves (2). The sphenoid sinus is directly medial to the optic canal and in approximately 5% of individuals, the bony wall separating these structures may be incomplete, putting these patients at high risk of complications (15). The differential diagnosis for intranasal destructive processes includes inflammatory, neoplastic, infectious, and idiopathic etiologies (Table 1) (16-21). The term ‘‘lethal midline granuloma,'' formerly used to encompass a range of conditions including Wegener granulomatosis, lymphoma, carcinoma, tuberculosis, and invasive fungal infection, is now considered outdated. A specific diagnosis should be made based on clinical and morphologic characteristics as well as diagnostic studies (22). 51 J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 Shen et al FIG. 2. Histopathology from the first biopsy shows necrotic nasal epithelium and an underlying subepithelial inflam-matory reaction composed of polymorphonuclear cells, eosinophils, lymphocytes, and plasma cells at different magnifications. (Hematoxylin and eosin.) FIG. 3. Histopathology from the third orbital procedure shows diffuse irregular collagen deposits with focal chronic inflammatory cells. Entrapped thick-walled blood vessels (white arrows) indicate the long-standing nature of the inflammation. The high-power magnification (lower image) shows proliferating fibroblasts (black arrows). Although there was marked cellular pleomorphism, no mitotic figures were seen. (Hematoxylin and eosin.) Clinical suspicion and a thorough history and physical examination are critical for accurate diagnosis. Laboratory workup can include ESR, renal function, ANA, RPR, nasal cultures, angiotensin-converting enzyme (ACE) level, and c-ANCA. Urine and serum toxicology studies may be helpful in cases of suspected cocaine abuse. Neuroimaging may facilitate demarcation of the extent of the nasal disease but may not be helpful in distinguishing between potential etiologies. For patients with significant sinonasal ulceration and necrosis, a biopsy should be performed. With intranasal cocaine use, biopsy of the affected tissue for histopathologic studies may reveal focal areas of chronic inflammation and necrosis, but typically will not show the necrotizing granulomas or vasculitis typical of Wegener granulomatosis (17). Flow cytometry 52 q 2009 Lippincott Williams & Wilkins Cocaine Abuse J Neuro-Ophthalmol, Vol. 29, No. 1, 2009 TABLE 1. Differential diagnosis of necrotizing sino-orbital inflammation Inflammatory (‘‘granulomatous'') diseases Wegener granulomatosis Sarcoidosis Relapsing polychondritis Systemic lupus erythematosus Mixed connective tissue disease Neoplastic diseases Nasal angiocentric lymphoma ( polymorphic reticulosis) Non-Hodgkin lymphoma including nasal-type natural killer/T-cell lymphoma (NK/T-cell lymphoma) Infectious diseases Recurrent bacterial sinus infections Syphilis Fungal infections of the orbit and paranasal sinuses including Fusarium and Mucormycosis Idiopathic disorders Idiopathic midline destructive disease Orbito-sino-nasal allergic fungal inflammation (20,21) and T-cell gene rearrangement studies may be helpful in ruling out lymphoma. Persistent investigation is often necessary to confirm a diagnosis in these difficult cases. Frequent biopsies and repeat laboratory studies may be necessary, especially because the diagnosis of Wegener granulomatosis can be difficult to make if the disease is confined to the sinonasal cavity (23,24). In our patient, histopathologic findings consisted of a wide range of inflammation, from necrotizing acute changes to chronic inflammation in a perivascular distri-bution with scattered dense fibrosis. The alternating areas of acute and chronic inflammation and focal dense fibrosis reflect the repetitive nature of the chemical insult by the cocaine abuse. As certain parts of the tissue attempt to heal with fibrosis, other areas become involved with newly developing acute ischemia and necrosis. This pattern of inflammation probably stimulates fibroblastic proliferation to produce irregular dense collagen fibers and atypical-appearing fibroblasts (Fig. 3). Such a pattern, seen most prominently in the second biopsy of our patient, raised the question of a low-grade fibrous tumor. Alexandrakis et al (5) reported a similar finding in their Case 1. In our patient, many pleomorphic fibroblasts were present, but no tumor atypia or mitotic figures were seen. In some areas, thick-walled capillaries were present within fibrous areas, but in other areas, the walls of similar-diameter vessels were not thickened. The presence of thick-walled vessels in fibroproliferative tissue is another indication of a long-standing inflammatory process affecting different regions of the tissue at different times. REFERENCES 1. Revis DR Jr, Seagle MB. Topical anesthetics, cocaine. Available at: http://www.emedicine.com/ent/topic384.htm. Accessed March 31, 2008. 2. Newman NM, DiLoreto DA, Ho JT, et al. Bilateral optic neuropathy and osteolytic sinusitis. JAMA 1988;259:72-4. 3. Smith JC, Kacker A, Anand VK. Midline nasal and hard palate destruction in cocaine abusers and cocaine's role in rhinologic practice. Ear Nose Throat J 2002;81:172-7. 4. Underdahl JP, Chiou AG. Preseptal cellulitis and orbital wall destruction secondary to nasal cocaine abuse. Am J Ophthalmol 1998;125:266-8. 5. Alexandrakis G, Tse DT, Rosa RH, et al. Nasolacrimal duct obstruction and orbital cellulitis associated with chronic intranasal cocaine abuse. Arch Ophthalmol 1999;117:1617-22. 6. Libman RB, Masters SR, de Paola A, et al. Transient monocular blindness associated with cocaine abuse. Neurology 1993;43:228-9. 7. Harper SJ, Jones NS. Cocaine: what role does it have in current ENT practice? A review of the current literature. J Laryngol Otol 2006; 120:808-11. 8. Vilela RJ, Langford C, McCullagh L, et al. Cocaine-induced oronasal fistulas with external nasal erosion but without palate involvement. Ear Nose Throat J 2002;81:562-3. 9. Talbott JF, Forti GK, Koch RJ. Midfacial osteomyelitis in a chronic cocaine abuser: a case report. Ear Nose Throat J 2001;80:738-40, 742-3. 10. Trimarchi M, Nicolai P, Lombardi D, et al. Sinonasal osteocartila-ginous necrosis in cocaine abusers: experience in 25 patients. Am J Rhinol 2003;17:33-4. 11. Kuriloff DB, Kimmelman CP. Osteocartilaginous necrosis of the sinonasal tract following cocaine abuse. Laryngoscope 1989;99: 918-24. 12. Sittel C, Eckel HE. Nasal cocaine abuse presenting as a central facial destructive granuloma. Eur Arch Optorhinolaryngol 1998;255: 446-7. 13. Goodger NM, Wang J, Pogrel MA. Palatal and nasal necrosis resulting from cocaine misuse. Br Dent J 2005;198:333-4. 14. Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions. Medicine (Baltimore) 2001;80:391-404. 15. Kline LB, ed. Basic and Clinical Science Course Section 5: Neuro-Ophthalmology. San Francisco: American Academy of Ophthalmology; 2007:9. 16. Pickens JP, Modica L. Current concepts of the lethal midline granuloma syndrome. Otolaryngol Head Neck Surg 1989;100: 623-30. 17. Sercarz JA, Strasnick B, Newman A, et al. Midline nasal destruction in cocaine abusers. Otolaryngol Head Neck Surg 1991;105: 694-701 18. Graper RG, Orenstein HH, Rohrich RJ, et al. Idiopathic midline granuloma-current classification and management controversies. Ann Plast Surg 1996;37:532-7. 19. Seyer BA, Grist W, Muller S. Aggressive destructive midfacial lesion from cocaine abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:465-70. 20. Chang WJ, Shields CL, Shields JA, et al. Bilateral orbital involvement with massive allergic fungal sinusitis. Arch Ophthalmol 1996;114:767-8. 21. Cruz AAV. Orbital inflammation and infection versus neoplasia. In: Karcioglu ZA, ed. Orbital Tumors: Diagnosis and Treatment. New York: Springer; 2005:325-327. 22. McDonald TJ. Nasal manifestations of systemic diseases. In: Cummings CW, Flint PW, Harker LA, et al., eds. Cummings Otolaryngology- Head and Neck Surgery. Vol 2. 4th ed. Philadelphia: Elsevier; 2005:934. 23. Borges A, Fink J, Villablanca P, et al. Midline destructive lesions of the sinonasal tract: simplified terminology based on histopathologic criteria. AJNR Am J Neuroradiol 2000;21:331-6. 24. Goldberg RA, Weisman JS, McFarland JE, et al. Orbital inflam-mation and optic neuropathies associated with chronic sinusitis of intranasal cocaine abuse. Arch Ophthalmol 1989;107:831-5. 53 |