| Title |
Impact of Low NOx Firing Conditions on Unburned Carbon Losses |
| Creator |
Veranth, John M.; Fletcher, Thomas H.; Pershing, David W.; Sarofim, Adel F. |
| Publisher |
University of Utah |
| Date |
1998 |
| Spatial Coverage |
presented at Maui, Hawaii |
| Abstract |
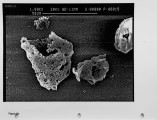
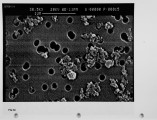
The unburned carbon in the fly ash from both laboratory and full-scale pulverized coal combustion contains both ultrafine soot particles and large char particles. Laboratory furnace data for bituminous coals show that the unburned carbon increases when staged combustion is used to reduce NOx emissions but the unburned carbon in lignite fly ash is low for both high-NOx and low-NOx combustion conditions. The carbon in the fly ash shows a bimodal distribution with the carbon concentration reaching a minimum in the 1-3 um aerodynamic diameter fraction. Electron microscope images confirm that the unburned carbon found in the fly ash over 10 um aerodynamic diameter is a mixture of soot aggregates and porous coal char particles. A method for quantitatively estimating the relative mass of soot and char in coal fly ash samples has been developed and validated. For the extreme low-NOx laboratory combustion conditions the furnace exit ash fly ash contains about 1 0% soot by weight, and this exit soot represents 0.2 to 0.6% of the fuel carbon. Refined samples of soot and char were separated from both laboratory fly ash and from fly ash obtained from commercial power plants. Preliminary data are reported on the surface area per unit mass, and low-temperaure reactivity of the separated fractions. These experimental methods and observations are being used to develop mechanistic hypotheses for large soot aggregate formation and survival, and provide a basis for further investigation. |
| Type |
Text |
| Format |
application/pdf |
| Language |
eng |
| Rights |
This material may be protected by copyright. Permission required for use in any form. For further information please contact the American Flame Research Committee. |
| Conversion Specifications |
Original scanned with Canon EOS-1Ds Mark II, 16.7 megapixel digital camera and saved as 400 ppi uncompressed TIFF, 16 bit depth. |
| Scanning Technician |
Cliodhna Davis |
| ARK |
ark:/87278/s6057jh7 |
| Setname |
uu_afrc |
| ID |
10392 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6057jh7 |