| OCR Text |
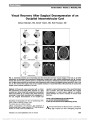
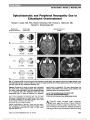
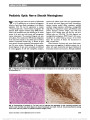
Show Tigers and Snakes in Neuro-Ophthalmology Harold E. Shaw Jr, MD In her book Eyes Wide Open: How to Make Smart Decisions in a Confusing World, Noreena Hertz tells the story of an experiment conducted by cognitive psychologists in which subjects were asked to view a variety of scenes and report what they saw (1,2). One of the scenes was of a tiger in a forest with a snake hidden in the background. Some subjects concentrated mainly on the tiger in the foreground and failed to see the snake. Others focused on the entire scene and did see the snake. Using this study as an analogy, Hertz emphasized that to make the best possible decisions, we need to see beyond the obvious, to see the snakes as well as the tigers. In the complex world of neuro-ophthalmology, we have our own versions of tigers and snakes. Consider the following cases. Case 1 An 89-year-old woman developed headaches. Her family informally asked a neurosurgeon for advice. Magnetic resonance imaging (MRI) of the brain was recommended, and it showed a mass in the right cavernous sinus characteristic of a meningioma (Fig. 1). Three weeks later, she experienced sudden loss of vision in her right eye, followed in 5 days by loss of vision in her left eye. She was referred for neuro-ophthalmologic evaluation and a history of malaise and jaw claudication was elicited. Examination revealed finger counting vision bilaterally, ropy temporal arteries, poorly reactive pupils, and bilateral optic disc swelling with hemorrhages (Fig. 2). Erythrocyte sedimentation rate was 121 mm/hr. A diagnosis of bilateral arteritic anterior ischemic optic neuropathy due to giant cell arteritis was made, and corticosteroid therapy was initiated. Her constitutional symptoms improved, but her vision re-mained unchanged, and she developed bilateral optic atrophy. Her intracranial meningioma remained stable until her death 2 years later from unrelated causes. Comment The finding of a brain tumor in this patient seemed to explain her headaches. Only when she lost vision in both eyes was she referred for neuro-ophthalmologic evaluation. Her headaches and other symptoms were then recognized to be manifestations of giant cell arteritis. Delayed diagnosis resulted in bilateral blindness. Her cavernous sinus meningioma was an incidental finding and never became symptomatic. FIG. 1. Case 1: Contrasted T1 axial magnetic resonance imaging shows a right parasellar mass, most likely a meningioma. FIG. 2. Case 1: Bilateral optic disc edema is present. Neuro-Ophthalmology Section, Jervey Eye Group, Greenville, South Carolina. The author reports no conflict of interest. Address correspondence to Harold E. Shaw Jr, MD, Neuro-Ophthalmology Section, Jervey Eye Group, 1 Doctors Drive, Greenville, SC 29605; E-mail: halshaw@gmail.com Shaw: J Neuro-Ophthalmol 2014; 34: 213-217 213 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Case 2 A 66-year-old man with slowly progressive bilateral loss of vision was referred for neuro-ophthalmologic evaluation. He had a history of heavy smoking, daily alcohol consump-tion, and a poor diet for many years. Examination revealed visual acuity of 20/400, right eye and 20/70, left eye, mild bilateral optic disc pallor, and bilateral cecocentral scotomas (Fig. 3). A diagnosis of nutritional optic neuropathy was made. Smoking cessation, alcohol abstinence, and improved nutrition were recommended, and he was treated with a daily multivitamin and weekly intramuscular injections of hydroxocobalamin. Over the next several months, his visual acuity progressively improved to 20/40, right eye and 20/60, left eye. Follow-up visual field testing unexpect-edly showed worsening field defects bilaterally (Fig. 4). Carotid angiography demonstrated a giant supraclinoid FIG. 3. Case 2: Kinetic visual fields demonstrate cecocentral scotoma in each eye. FIG. 4. Case 2: Follow-up perimetry shows nerve fiber bundle defects with relative central depression bilaterally. FIG. 5. Case 2: Lateral projection of carotid angiogram re-veals a giant supraclinoid internal carotid artery aneurysm. 214 Shaw: J Neuro-Ophthalmol 2014; 34: 213-217 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. internal carotid artery aneurysm (Fig. 5). He underwent left extracranial-intracranial bypass surgery with clipping of the aneurysm that was complicated postoperatively by a stroke. The patient was left with a complete right homonymous hemianopia. However, visual acuity improved to 20/25 in each eye and remained stable thereafter. Comment This patient had classic findings of nutritional optic neurop-athy. So certain was the diagnosis that initially neuroimaging was not ordered. Had it been, his aneurysm would have been the focus of concern, and his nutritional amblyopia might not have received the attention that it deserved. The first diagnosis was correct, but it did not tell the whole story. Follow-up visual field testing identified changes that led to the diagnosis and treatment of his intracranial aneurysm. Case 3 A 69-year-old woman had sensorineural hearing loss in her left ear and decreased peripheral vision in her right eye. Brain MRI revealed left cerebellopontine angle and right medial sphenoid lesions consistent with meningiomas (Fig. 6A, B). Her medical history was significant for systemic hyperten-sion, atrial fibrillation, anemia, and migraine. Her mother and 2 sisters had glaucoma. On neuro-ophthalmologic exam-ination, visual acuity was 20/25, right eye and 20/20, left eye. In her right eye, she had mild dyschromatopsia, a relative afferent pupillary defect, and a predominantly inferior nerve fiber bundle visual field defect (Fig. 7). Intraocular pressures were 16 mm Hg in the right eye and 15 mm Hg in the left eye. Ophthalmoscopy showed prominent optic disc cupping bilaterally with focal thinning of the superotemporal neural rim of the right optic disc. Corresponding superotemporal retinal nerve fiber layer thinning was demonstrated by optical coherence tomography (Fig. 8). The patient had radiation treatment for her meningiomas, and she was treated as a nor-mal tension glaucoma suspect with latanoprost ophthalmic drops. One year later, her visual function was unchanged. Comment This patient had intracranial meningiomas, one of which compressed her right optic nerve. She also had findings consistent with normal tension glaucoma. Missing that diagnosis could have resulted in insidious loss of vision mistakenly attributed to compressive optic neuropathy. This case illustrates the potential dilemmas and pitfalls associated with the diagnosis and management of concur-rent conditions. Occam's razor is a precept that states: "Entities should not be multiplied beyond necessity" (3). This principle has been expressed in various ways by Aristotle, Ptolemy, Maimonides, Thomas Aquinas, and others, including 14th century English philosopher and theologian William of Ockham (or Occam), who wrote: "Pluralitas non est ponenda sine necessitate," translated to "plurality should not be posited without necessity" (3-7). In 1852, centuries after Ockham's death, this principle of parsimony was referred to in the works of Scottish metaphysical philosopher William Hamilton as Occam's razor (3,6). FIG. 6. Case 3: Postcontrast T1 coronal magnetic resonance imaging shows (A) left cerebellopontine angle and (B) right medial sphenoid meningiomas (arrows). FIG. 7. Case 3: Automated visual field testing shows field loss in the right eye. Shaw: J Neuro-Ophthalmol 2014; 34: 213-217 215 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. In medical practice, Occam's razor is a useful heuristic that pertains to diagnostic parsimony. For any given illness, a unifying diagnosis to explain a patient's symptoms is pref-erable to multiple diagnoses-do not make 2 diagnoses when one will do. Noble David, MD, succinctly described Occam's razor as "one disease to a customer" (8). The med-ical counter-argument to Occam's razor is Hickam's dictum. Attributed to mid-20th century Duke medicine professor John B. Hickam, it states: "Patients can have as many dis-eases as they damn well please" (7-9). These counterbalancing philosophies are relevant to neuro-ophthalmology, as evidenced by the cases described above. Each patient had 2 important diseases, one of which was more obvious but not necessarily more significant, than the other. Differentiating patients who conform to Occam's razor from those who fulfill Hickam's dictum can be chal-lenging and subject to cognitive biases (10-13). Cognitive biases are predictable and recurring thinking errors that we all make when we acquire and process information (14). It is helpful to consider cognitive biases in the context of a dual thinking system that has intuitive (System 1) and analytical (System 2) components (13-15). Intuitive thinking is fast, automatic, and unconscious; ana-lytical thinking is slow, deliberate, and conscious (13-15). Although intuitive thinking is highly effective and essential to our daily decision-making, it is in this mode that most biases and other cognitive failures occur (12). In neuro-ophthalmology, as in other disciplines, an appropriate blend of intuitive and analytical thinking would seem to provide the best foundation for optimal decision-making (13,16). FIG. 8. Case 3. There is increased cupping of both optic discs with thinning of the superotemporal neural rim of the right optic disc. This is confirmed on optical coherence tomography. 216 Shaw: J Neuro-Ophthalmol 2014; 34: 213-217 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Of the many cognitive biases that are known, among the most important ones that affect our clinical decision-making are anchoring, availability, confirmation, framing, unpacking, and premature closure (10,11). Anchoring bias is the tendency to rely too heavily on certain information early in the diagnostic process, to be overly influenced by first impressions, and thereby to limit consideration of alternative explanations. Availability bias is the tendency to overestimate the likelihood of events based on what we have recently seen or experienced and what we most remember. Confirmation bias is the tendency to focus on information that supports, rather than refutes, our preconceptions. Framing bias occurs when different con-clusions are drawn from the same information, depending on how that information is presented. The unpacking principle refers to failure in eliciting all relevant informa-tion, which may result in other significant possibilities being overlooked. Premature closure is the tendency to suspend the decision-making process too soon, accepting a diagnosis before it has been confirmed. Said in another way, "When the diagnosis is made, the thinking stops." In each of the cases described, various combinations of these thinking er-rors occurred or potentially could have occurred. A number of strategies to eliminate or reduce the influence of cognitive biases in clinical decision-making have been proposed; chief among them are having greater appreciation and awareness of biases, improving medical education, improving critical thinking skills, and thinking about one's own thinking, a process known as metacognition (10,12,17,18). Each of these debiasing strategies requires vigilance and sustained commitment. In neuro-ophthalmology, we see a wide array of complex cases that are time- and resource-intensive, cognitively challenging, and beset with uncertainty. In some cases, the obvious diagnosis might not be the most important one. We always should be mindful of the thinking traps to which we are susceptible. A thorough history, meticulous examination, and conscientious follow-up are timeless, indispensible tools for the neuro-ophthalmologist. When these tools are combined with strategies to avoid thinking errors in clinical decision-making, we minimize the likelihood of misdiagnosis and improve our quality of care. We increase our chances of seeing the snakes as well as the tigers. REFERENCES 1. Hertz N. Eyes Wide Open: How to Make Smart Decisions in a Confusing World. New York, NY: HarperCollins Publishers, 2013. 2. Chua HF, Boland JE, Nisbett RE. Cultural variation in eye movements during scene perception. Proc Natl Acad Sci U S A. 2005;102:12629-12633. 3. Kaye S. William of Ockham. Available at: http://www.iep.utm. edu/ockham/. Accessed February 28, 2014. 4. Hiroshi S. What is Occam's razor? Available at: http://math. ucr.edu/home/baez/physics/General/occam.html. Accessed February 28, 2014. 5. Carroll RT. Occam's razor. Available at: http://skepdic.com/ occam.html. Accessed February 28, 2014. 6. Wikipedia. Occam's razor. Available at: http://en.wikipedia. org/wiki/Occam's_razor. Accessed February 28, 2014. 7. Maloney WJ. Occam's Razor and Hickam's Dictum. The transformation of a theoretical discussion into a modern and revolutionary tool in oral diagnostics. Dentistry. 2011;2: WMC001914. 8. Trobe JD. Noble J. David, MD, Reminisces. J Neuroophthalmol. 2002;22:240-246. 9. McIntosh HD. Memorial. John Bamber Hickam, MD. Trans Am Clin Climatol Assoc. 1971;82:lx-lxii. 10. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. 11. Groopman J. How Doctors Think. New York, NY: Houghton Mifflin Company, 2007. 12. Croskerry P. From mindless to mindful practice-cognitive bias and clinical decision making. N Engl J Med. 2013;368: 2445-2448. 13. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22 (suppl 2):ii58-ii64. 14. Kahneman D. Thinking, Fast and Slow. New York, NY: Farrar, Straus and Giroux, 2011. 15. Evans JSBT, Stanovich KE. Dual-process theories of higher cognition: advancing the debate. Perspect Psychol Sci. 2013;8:223-241. 16. Van Stavern GP. Neuro-ophthalmology clinical decision making. North American neuro-ophthalmology Society Annual Meeting Syllabus. 2011;361-368. Available at: http:// content.lib.utah.edu/cdm/ref/collection/ehsl-nam/id/1252. Accessed February 28, 2014. 17. Graber ML, Kissam S, Payne VL, Myer AND, Sorensen A, Lenfestey N, Tant E, Henriksen K, LaBresh K, Singh H. Cognitive interventions to reduce diagnostic error: a narrative review. BMJ Qual Saf. 2012;21:535-557. 18. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72. Shaw: J Neuro-Ophthalmol 2014; 34: 213-217 217 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |