| OCR Text |
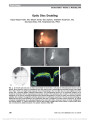
Show Cognition and Eye Movements: Assessment of Cerebral Dysfunction Owen B. White, MBBS, MD, PhD, FRACP, Joanne Fielding, PhD Background: Many neurological disorders show deficits in ocular motor function. In the past, evaluation has been limited to assessing abnormalities largely generated by pathology of the brainstem andcerebellum. In disorders that primarily or substantially, affect the cerebral hemispheres, disruption of cognitive processes occur, often early in the clinical course. While neuropsychological testing traditionally is used to measure cognitive performance, the cerebral influences on the ocular motor system provides another quantitative paradigm. This review explores the relationship between cognitive sensory processing and execution of planned ocular motor tests in Parkinson's disease, Huntington's disease and multiple sclerosis and explores areas of clinical utility. Methods: Review of the literature regarding cognitive and ocular motor abnormalities in neurological disease. Results: The literature indicates that in systems degener-ation there are abnormalities of cognitive processing, defined both by conventional behavioural testing and by assessment of cognitive function utilizing ocular motor studies, which characterise those processes. Moreover, in diffuse disease, in processes such as multiple sclerosis, the assessment of cognitive processes involved in ocular motor function may well provide an added level of sensitivity indicating more widespread pathology than would be apparent with conventional clinical assessment. Conclusions: Assessment of cognitive function in the ocular motor system may provide insight into cerebral function, in health and disease, and may provide both diagnostic information and permit quantification of deficit in future. Journal of Neuro-Ophthalmology 2012;32:266-273 doi: 10.1097/WNO.0b013e3182688230 © 2012 by North American Neuro-Ophthalmology Society The eye is a window to the world but, from the neuro-ophthalmologic perspective, the eye and its purposeful movements can also represent a window into higher func-tions of the brain. Quantification of cerebral function using neurobehavioral tools is the gold standard, but it can be difficult to assess in some patients whose subjective responses may not always be reliable. Accurate assessment of cognitive function is critically important in chronic, pro-gressive neurological disorders, in which diagnosis and optimal treatment is uncertain. Cerebral pathology in neurodegenerative disorders may be focal, multifocal, or diffuse. Many of these disorders show dysfunction in the brainstem, pyramidal system, and cortical areas of cognitive function, all of which may affect clinical assessment of eye movements. It would be clinically desirable to parse out the various components of dysfunc-tion in the brainstem, pyramidal system, and cortical levels using specific eye movement testing paradigms. Such an approach would be beneficial in assessing conditions such as multiple sclerosis (MS) (1) and Parkinson disease (2), in which cognitive abnormalities can be identified at an early stage, even when there is no evidence of dementia. Unlike humans, responses to stimuli in lower order organisms may be "hard wired" and invariable. Frogs, for example, respond to a fly-sized object moving within their field of vision with a tongue flick. The result is dinner. After cutting the optic nerve, inverting the eye and allowing full regeneration, the response is now a tongue flick into the dirt. The result is disappointment (3). With the development of the cerebrum in phylogenetically higher order animals, volun-tary motor activity becomes less hard wired and requires cog-nitive integration with reflexive motor activity. This is particularly true in the ocular motor system, with voluntary, cognitively directed (top down) eye movements being inte-grated with reflexive (bottom-up) activity to enable scanning of the environment and appropriate response to visual stimuli. The cognitive control of eye movements requires synchronization of circuits between frontal and parietal cortex as well as subcortical nuclei to produce appropriate context-specific responses. Direct projections exist, permit-ting serial processing of afferent-efferent responses. There Departments of Neurology and Medicine and the Melbourne Brain Centre, the University of Melbourne at Royal Melbourne Hospital, Melbourne, Victoria, Australia; and Department of Neuropsychology, School of Psychology and Psychiatry, Monash University, Clayton, Victoria, Australia. The authors report no conflicts of interest. Address correspondence to Owen B. White, Department of Neurology, Royal Melbourne Hospital, Grattan Street, Parkville, Victoria, 3050, Australia. E-mail: owen.white@mh.org.au 266 White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 State-of-the-Art Review Section Editors: Grant T. Liu, MD Randy H. Kardon, MD, PhD Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. are also parallel projections to single sensory mode associa-tion cortices and to multimodal association sensory cortices, both of which feed forward (and backward) providing com-plex parallel processing and parsing of preferred sensory responses. All this information is integrated with voluntary willed (top-down) activity, before a motor action is gener-ated. Moreover, there are circuits through the basal ganglia and back to cortex via thalamus, for the somatic motor system, and to the superior colliculus via substantia nigra pars reticularis, for the ocular motor system (4) (Fig. 1). This system of intermediary processing, bridging the gap between sensation and action, including a range of processes like memory, attention, language, thought and emotion, is what we recognize as cognition (3). Many aspects of cognition not only depend upon processing of real-time domain sensory events but also the interpretation of these events on the basis of previous experience. This involves circuits through frontal and prefrontal cortex with wide-ranging inputs from other areas (5,6). In reality, cognition is the interaction of top-down with bottom-up inputs, with reciprocal modulation both in real time and over time, with the resultant motor responses. This review explores the evaluation of cognitive processes in the ocular motor system in Parkinson disease, primarily a basal ganglia disorder, Huntington disease, a degenerative disease of basal ganglia and cortex, and MS, a widespread multilesion demyelinating disorder. CLINICAL AND ANATOMICAL ISSUES Neuro-opthalmologists are experienced in the diagnosis of disease based upon characteristic eye movement abnormal-ities. Almost exclusively, this involves extraocular muscles, cranial nerves, peripheral nerves, brainstem, and cerebellum. In general, we do not use eye movement abnormalities to any substantial degree in the evaluation of cerebral hemi-spheric pathology. There is a developing literature regarding focal hemi-spheric lesions of cortex and deep nuclei and white matter, which produce specific abnormalities of cognitive control of eye movements. Presumably, focal abnormalities either interrupt crucial pathways or damage nodal sites of convergence and cognitive parsing of data. Documentation of such deficits may provide insight into the pathophysiol-ogy of neurodegeneration and multifocal disease. The ocular motor networks substantially overlie the hemi-spheric attentional systems in frontal, temporal, and parietal lobes (7), interacting at many levels (8-17). Cognitive pro-cesses are most readily examined with saccades. Smooth pur-suit eye movements, in the past considered to have a different control system, have more recently been postulated as merely a different outcome of the same cognitive processes (18,19). Saccadic testing paradigms include shifting of attention, en-dogenous, top-down voluntary saccades, reflexive, bottom-up saccades, working memory, spatial and temporal, and inhibi-tion of nonsalient distractors. Documentation of performance can provide insight into hemispheric structural integrity. In broad terms, frontal cortex regions of interest include the frontal eye fields (FEFs), supplementary eye fields (SEFs), and dorsolateral prefrontal cortex (DLPFC), with inputs from orbital and medial frontal cortex to these areas (6). Studies of the FEFs indicate they have a role in the generation of "top-down" driven saccades, such as those spontaneously exploring contralateral hemispace, anticipatory saccades, and saccades to remembered targets (20-22). Antisaccades are a special case requiring inhibition of a reflexive saccade and reprogramming of an internally driven saccade to the opposite hemifield involving widespread activation of cortex (23-25). Lesions of the SEFs do not affect the dynamics of bottom-up reflexive saccades but seem more involved with temporal sequencing of combinations of movements. Imaging studies demonstrate activation during voluntarily generated and memory-guided saccades as well as with antisaccades (26). FIG. 1. Neural regions involved in the control of saccadic eye movement. CN, caudate nucleus; DLPFC, dorsolateral prefrontal cortex; FEF, frontal eye field; GPe, globus pal-lidum externa, GPi, globus pallidus interna; INC, interstitial nucleus of Cajal; IPL, inferior parietal lobule; LGN, lateral geniculate nucleus; LIP, lateral intraprietal sulcus; NRTP, nucleus reticularis tegmenti pontis; PEF, parietal eye field; PPC, posterior parietal cortex; PPRF, paramedian pontine reticular formation; Pre-SMA, prefrontal supplementary motor area; riMLF, rostral interstitial nucleus of the median longitudinal fasciculus; RIP, nucleus raphe interpositus; SEF, supplementary eye field; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticularis; STN, subthalamic nucleus; TPJ, temporoparietal junction (inferior parietal lobule, IPL/superior temporal gyrus, STG). White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 267 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. DLPFC plays a decisive role in saccade generation, integrating working memory and receiving data from mesial and orbitofrontal cortex, implying a role in inhibiting unwanted saccades as well (6,26,27). Reflexive saccades appear to be more a function of the parietal eye field in the posterior parietal cortex (24,26) with diversion of attention being signaled in this region, and attention to regions of space being particularly dependent on the supramarginal gyrus (28). In addition, there is parallel and serial communication of central regions within the frontal, temporal, and parietal lobes with the basal ganglia. The cortical areas are nodes of activity, with confluence of signals, in widely ramifying hemispheric networks. Pathology at various levels within a particular circuit will result in errors of performance in specific parameters or a range of parameters, as well as nonspecific errors of performance. NEUROPSYCHOLOGICAL ASSESSMENT Neuropsychological evaluation measures activity in wide-spread cerebral circuitry not readily assessed with standard clinical examination techniques or neuroimaging studies. Methods of evaluation primarily document deficits in plan-ning, working memory, attention, problem solving, mental flexibility, multitasking, initiation, and monitoring of actions. Testing is complex and involves cognitive functions without directly relating them to output functions (Table 1). For example, deficits in Parkinson disease may span the full spectrum from mild executive dysfunction and memory failure to overt dementia associated with severely impaired thought processes and manipulation of acquired knowledge (2,29-31). The severity of cognitive impairment correlates with more advanced motor dysfunction and particularly with postural instability of gait (31). Cognitive dysfunction has been widely recognized as an independent entity in Parkinson disease (32) that correlates over time with pro-gression of motor dysfunction. There remains uncertainty whether this correlation is an epiphenomenon or plays a dis-tinct role in the generation of motor deficits (33,34). Huntington disease shares some characteristics with Parkinson disease, being a degenerative disorder with widespread basal ganglia pathology, but differs in that there is also extensive cortical degeneration. Cognitive dysfunc-tion includes impaired attention (35), language (36) and memory disturbances (37) and reduced perceptual process-ing speed and memory. Cognitive abnormality has been associated with structural abnormality. For example, impair-ment of executive function correlates with atrophy of the striatum and insula on magnetic resonance imaging (MRI) (38). Neuropsychological profiling has been used to identify patients at an early stage (39) and investigated as a means of TABLE 2. Ocular motor testing of cognitive function Cognitive Domain Ocular Motor Paradigms Attention Visually/symbolically cued saccades, gap-overlap task Memory Memory-guided saccades, memory-guided sequences of saccades Executive function Antisaccades Visually/symbolically cued saccades, participants fixate a central target and are presented with a cue indicating the possible location of an upcoming target. A target then appears, which is either congruent or incongruent with the preceding cue and the subsequent saccade is then evaluated. Gap-overlap task, participants fixate a central target that is extinguished either before or after the presentation of a peripheral target. They make a saccade to the peripheral target on presentation. Memory-guided saccades, participants fixate a central target while a peripheral target flashes briefly. After a variable delay, the central target extinguishes and participants make a saccade to the remembered previously illuminated target position. Memory-guided sequences, targets are present on the screen. Participants must then memorize the sequence in which they illuminate and subsequently make saccades sequentially to those targets on cue. Antisaccades, participants fixate a central target. A peripheral target appears and they make a saccade of equal amplitude in the opposite direction. TABLE 1. Common tests of neuropsychological function Cognitive Domain Tests Attention Symbol Digit Modalities Test, Paced Auditory Serial Addition Test Memory Digit span, brief visuospatial memory test Executive function Stroop, Wisconsin card sorting task Symbol Digit Modalities Test, a simple substitution task. Using a reference key, the participant has 90 seconds to pair specific numbers with given geometric figures. Paced Auditory Serial Addition Test, participants are given a number every 3 seconds and are asked to add the number they just heard with the number they heard before. Digit Span, participants are presented with a series of digits (e.g., ‘8, 3, 4') and must immediately repeat them back. If they do this successfully, they are given a longer list (e.g., ‘9, 2, 4, 0'). The length of the longest list a person can remember is that person's digit span. Brief Visuospatial Memory Test, participants view a number of geometric patterns on the stimulus page for 10 seconds and are then asked to draw as many of the figures as possible in their correct location on a page in the response booklet. Stroop, the written color name differs from the color ink it is printed in, and the participant must either say the written word or name the ink color. Wisconsin Card Sorting Task, stimulus cards are matched by color design or quantity. The participant is not told how to match the cards; however, he or she is told whether a particular match is right or wrong. During the course of the test, the matching rules are changed and the time taken for the participant to learn the new rules, and the mistakes made during this learning process are analyzed to arrive at a score. 268 White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. monitoring the development of disease (40). Unfortunately, such deficits can be seen in lesional disease as well and, as such, the value of a profile may not be so much in diagnosis but in establishing a profile in time for subsequent evalua-tion of progression. Cognitive deficits occur early in the course of clinically definite MS, at which time there may be minimal evidence of impairment as measured by the routinely used Kurtzke Expanded Disability Scale Score (EDSS) (41-43). In larger studies, cognitive deficits have been recognized in 45%-60% of patients across the spectrum of disease and have been shown to progress with the course of the disease, predicting decline in clinical performance and correlating, to some degree, with MRI changes (44-47). Much attention has been paid to neuropsychological impairment in traumatic brain injury, particularly with the development of the Ruff Neurobehavioral Inventory (48). Differences in self-perception of deficit have been demon-strated between those patients with mild injury and those with moderate to severe injury (49). Patients with milder traumatic brain damage typically demonstrate more atten-tional deficits, while more severely injured patients show difficulties with memory and learning. Studies have shown that conventional MRI of the brain does not define the widespread nature of white matter and cortical pathology for patients with moderate to severe injury, although such pathology is demonstrated by fractional anisotropy and dif-fusivity studies (50). COGNITION AND THE OCULAR MOTOR SYSTEM Movement of the eyes requires resolution of the potential conflict between top-down cognitive activity and bottom-up reflexive responses. This is the conflict between voluntary scanning of the environment and a response to a visual or auditory stimulus attracting attention. In a well-functioning system, information projects from visual cortex to association cortex, with subsequent parallel and serial projections to premotor and motor cortex. There are also numerous reciprocal cortical-basal ganglia connections. The final common output is a product of synaptic function at multiple levels, producing a balance of information resulting in excitation or inhibition of neuronal activity. In patho-logic states, abnormalities at different levels may produce characteristic, and possibly pathognomonic, patterns of dys-function. With multilesion disease, there may be an accu-mulation of deficits with increasing burden of disease. Investigation and evaluation of the cognitive processing involved in the generation of ocular motor responses permit correlation of motor dysfunction with cognitive deficits (Table 2). In particular, abnormalities of attention, including inhibition, working memory and executive function, may not merely be epiphenomena but may actually cause ocular mo-tor abnormalities (Fig. 2) (51-56). Such motor deficits may include delayed, slow, or hypometric saccades, as seen in Parkinson disease, or hyperactive saccadic function seen in Huntington disease. These changes may not only characterize disease processes, particularly those with well-defined systems pathology, but also document staging of disease. OCULAR MOTOR COGNITIVE FINDINGS IN NEUROLOGIC DISEASE A comprehensive literature review of all neuropathologic states with abnormal eye movement is beyond the scope of this review. As stated above, we will restrict the discussion to FIG. 2. Representation of target movement paradigms. Solid lines represent target position and dotted lines rep-resent eye position. 1. For the "GAP" paradigm, participants fixate the central position beyond the time the central target extinguishes, and then make a saccade to the eccentric target when it illuminates. A, the gap. For the "OVERLAP" paradigm, both central fixation and peripheral targets over-lap, but participants must make a saccade to the peripheral target immediately on presentation. This examines atten-tional engagement and disengagement of the central target. 2. For the " MEMORY-GUIDED" (M-G) saccades, participants fixate a central target. An eccentric target flashes briefly. When the central target extinguishes, they must make a saccade to the remembered position. A, latency, B and D, final eye position; C, error. This examines inhibitory function and spatial working memory. 3. For the "ANTISACCADE" protocol, a target appears and participants must make a saccade to the equal and opposite spatial location. A, latency; B, amplitude; C and F, final eye position; D, error latency; E, error correction. This examines inhibitory control and executive function. White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 269 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Parkinson disease and Huntington disease as system degenerations, MS, and traumatic brain injury (Table 3). Parkinson Disease Abnormalities of eye movement have long been recognized in Parkinson disease (57,58). There is general agreement that patients with Parkinson disease tend to make a series of hypometric saccades to achieve targets (51,59) (Table 3). There is some dispute as to whether latencies are always normal or abnormal and whether saccadic velocities are slowed (59-64). Ocular motor abnormalities in patients with Parkinson disease include slowed movement initiation, impaired executive function manifested as impaired generation of voluntary movements, and suppression of inappropriate reflexive movements (51,55,56,65-67). Abnormalities of remembered saccades previously have been attributed to a need for a visual target to make accurate saccades (68), implying visual feedback as a requirement for accurate per-formance right up until the time of saccade generation. This suggests that working memory is profoundly impaired. Investigation of memory-guided saccades (67), and the tem-poral effects of providing visual cue to direct attention before making a saccade, demonstrates a complex abnormal-ity of attention and working memory (56). Purely self-paced saccades, wherein 2 targets remain illuminated and participants saccade between them, are normal. These probably utilize direct frontocollicular path-ways bypassing the basal ganglia (69). However, studies of visually guided eye movements have shown complex effects of cues (valid cues truly indicating direction of target move-ment and invalid being misleading) and distractor stimuli on both saccades and smooth pursuit (52,65-67,70). For exam-ple, during presentation of smooth pursuit stimuli, we have shown differential effects of distractors in the hemifield ipsilateral to target movement versus those in the contralat-eral hemifield (67). Also, spatially invalid cues have a substan-tial effect on saccade latency compared with valid cues (56). Variation of ocular motor deficits seen in patients with Parkinson disease is explicable if one assumes that not all dopaminergic neurons in the substantia nigra pars compacta deteriorate at the same rate and not all patients are tested at the same stage of the disease. With progression of the disease, more characteristic eye movement abnormalities to specific tasks may develop. There are complex relationships between how well an object of regard is recognized within its visual environment including spatial characteristics of the target and competing targets (distractors) and the task the subject is being asked to perform. For example, in early Parkinson disease there is difficulty in performing antisaccades (Fig. 2). An antisaccade requires suppression of a reflexive saccadic response to a newly appearing object of regard and internal generation of a saccade in the opposite direction and of the same dis-tance from central fixation, in the absence of a target (51). Huntington Disease Huntington disease, commonly thought of as a basal ganglia disorder, has more complex pathology, with substantial cortical pathology becoming evident as the disease pro-gresses. The hallmark ocular motor abnormalities are difficulty in initiating saccades accompanied by saccadic intrusions (fixation instability) (71-74) and an increased tendency to make inappropriate saccades in response to targets and instructions (Table 3). Increased errors (either anticipatory or in response to a nontarget stimulus), increased latency, and greater variability of responses have been identified in antisaccade and memory-guided proto-cols (75). Complex spatial (55) and temporal (56) effects of cuing have been demonstrated in Huntington disease. This may be an effect of the basal ganglia pathology as seen in Parkinson disease, although there is a different ocular motor abnormality in each disorder. Patients with Parkinson dis-ease demonstrate increased inhibitory output from the basal ganglia and patients with Huntington disease show just the opposite-facilitation of saccades (56,65). This may be the TABLE 3. Summary of eye movement findings in Parkinson disease, Huntington disease, and multiple sclerosis Process Clinical Features Clinical Eye Movements Pathological Eye Movements Parkinson disease Bradykinesia, rigidity; tremor, impaired multitasking, cognitive impairment Hypometric and multistep saccades, long latencies, slowed saccades, variability, saccadic smooth pursuit Antisaccade errors, impaired memory-guided saccades, impaired endogenous cuing of saccades, impaired suppression of distractor stimuli Huntington disease Chorea, bradykinesia, dementia Intrusive saccades to irrelevant stimuli, impaired fixation, slowed saccades, impaired smooth pursuit Antisaccade errors, prolonged saccade latencies, impaired memory-guided saccades, impaired endogenous cuing; abnormal trajectory responses to distractor stimuli Multiple sclerosis Multiple motor and sensory deficits, cognitive impairment Internuclear ophthalmoplegia, spontaneous nystagmus, impaired smooth pursuit Antisaccade errors, impaired memory-guided saccades 270 White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. result of the differing effects these disorders have on inhib-itory and facilitatory pathways through the basal ganglia. Functional MRI studies performed during saccades to targets and antisaccades define characteristic patterns of activity in normal subjects. This includes fronto-parieto-subcortical network of FEFs, SEFs, DLPFC, ventrolateral prefrontal cortex, posterior parietal cortex, supram-arginal gyrus, striatum, thalamus, and cerebellum (25). This widespread activity also occurs in patients with Hun-tington disease, but with progression of disease and an increase in antisaccade errors, there is loss of activity in pre-supplementary and dorsal anterior cingulate regions (76). In these patients, particularly with antisaccade error, activity is more widespread, indicating compensatory activation of cor-tex not normally involved in error monitoring (76). Multiple Sclerosis Early diagnosis of MS is important because therapy with immune modulating agents improves long-term outcomes. With growing awareness that cognitive dysfunction is a "silent" process in patients with MS, detection of impaired cognitively mediated ocular motor may provide one method of establishing early diagnosis. Examination of antisaccade performance has been shown to be a sensitive marker of cerebral cognitive dysfunction in patients with early disease (77,78) (Table 3). Over a 2-year period, during which there was no change in EDSS, an increase in saccade latency and error rate has been shown to correlate with worsening in scores on Paced Auditory Serial Addition Test, considered the reference task for the cognitive evaluation of MS patients. Interestingly, impaired antisaccade performance also has been found in patients with a clinically isolated syndrome, suggestive of more wide-spread CNS dysfunction. In MS patients, visually guided saccades, in the presence of randomly presented distractor stimuli, shows abnormal function (79). MS patients also are less accurate and make more errors with memory-guided saccades (80), suggesting impaired working memory. These findings probably reflect abnormality in the extensive circuitry required for the inhibitory function of attentional control as well as working memory and programming of saccades. Such circuitry involves long range projections between parietal cortex and prefrontal cortex and corticobasal connections (Fig. 1). Traumatic Brain Injury In moderate traumatic brain injury, where conventional MRI studies are normal, more detailed imaging of axon bundles using fractional anisotropy and diffusivity demon-strate widespread disruption of white matter tracts. The use of eye movement paradigms to assess cognitive processes of working memory and attentional control could provide an opportunity to evaluate deficits in patients at an early stage and may be a fertile area for future research (81). It remains possible that early introduction of cognitive retraining programs may enhance recovery in patients with mild and moderate traumatic brain injury. SUMMARY Tests of cognition provide evidence of more widespread pathology in a number of processes that affect the brain, including degenerative diseases, autoimmune disorders and, potentially, trauma. The assessment of such tests is rightly within the domain of neuro-ophthalmology. It is a small step to extend evaluation from the eye to the brainstem and on to cognitive cerebral function. We measure individual eye movements and parameters of eye movements.We document abnormalities of eye movements unique to brainstem dys-function. Evaluation of cognitive processes, dependent on cerebral hemispheric circuitry, would seem a logical next step in terms of understanding central nervous system dysfunction. REFERENCES 1. Amato MP, Zipoli V, Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci. 2006;245:41-46. 2. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200-1213. 3. Mesulam MM. From sensation to cognition. Brain. 1998;121:1013-1052. 4. Obeso JA. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord. 2008;23:s548-s559. 5. Mesulam M. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Ann Neurol. 2008;64:367-378. 6. Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319-330. 7. Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761-773. 8. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201-215. 9. Walker R, Husain M, Hodgson TL, Harrison J, Kennard C. Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia. 1998;36:1141-1159. 10. Muggleton NG, Chen CY, Tzeng OJ, Hung DL, Juan CH. Inhibitory control and the frontal eye fields. J Cogn Neurosci. 2010;22:2804-2812. 11. Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925-931. 12. Watanabe M, Munoz DP. Probing basal ganglia functions by saccade eye movements. Eur J Neurosci. 2011;33: 2070-2090. 13. Heide W, Kompf D. Combined deficits of saccades and visuo-spatial orientation after cortical lesions. Exp Brain Res. 1998;123:164-171. 14. Heide W, Kurzidim K, Kompf D. Deficits of smooth pursuit eye movements after frontal and parietal lesions. Brain. 1996;119:1951-1969. 15. Lekwuwa GU, Barnes GR. Cerebral control of eye movements. II. Timing of anticipatory eye movements, predictive pursuit and phase errors in focal cerebral lesions. Brain. 1996;119: 491-505. White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 271 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 16. Lekwuwa GU, Barnes GR. Cerebral control of eye movements. I. The relationship between cerebral lesion sites and smooth pursuit deficits. Brain. 1996;119:473-490. 17. Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, Morgan PS, Jackson S, Rees G, Husain M. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia. 2007;45:997-1008. 18. Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol. 2004;91:591-603. 19. Krauzlis RJ, Basso MA, Wurtz RH. Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol. 2000;84:876-891. 20. Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI. Cortical control of saccades. Ann Neurol. 1995;37:557-567. 21. Fukushima J, Fukushima K, Miyasaka K, Yamashita I. Voluntary control of saccadic eye movement in patients with frontal cortical lesions and parkinsonian patients in comparison with that in schizophrenics. Biol Psychiatry. 1994;36:21-30. 22. Guitton D, Buchtel HA, Douglas RM. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp Brain Res. 1985;58:455-472. 23. Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, Taira M, Kojima T. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131:147-155. 24. McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255-270. 25. Ettinger U, Ffytche DH, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cereb Cortex. 2008;18:1148-1159. 26. Milea D, Lobel E, Lehericy S, Pierrot-Deseilligny C, Berthoz A. Cortical mechanisms of saccade generation from execution to decision. Ann N Y Acad Sci. 2005;1039:232-238. 27. Schall JD. Weighing the evidence: how the brain makes a decision. Nat Neurosci. 1999;2:108-109. 28. Perry RJ, Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123:2273-2288. 29. Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson's disease. The cortical focus of neostriatal outflow. Brain. 1986;109:845-883. 30. Taylor AE, Saint-Cyr JA, Lang AE, Kenny FT. Parkinson's disease and depression. A critical re-evaluation. Brain. 1986;109:279-292. 31. Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middelkoop HA, van Hilten JJ. Cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:1182-1187. 32. Bodis-Wollner I. Neuropsychological and perceptual defects in Parkinson's disease. Parkinsonism Relat Disord. 2003;9: 83-89. 33. Domellof ME, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson's disease. Mov Disord. 2011;26:2183-2189. 34. Fok P, Farrell M, McMeeken J. The effect of dividing attention between walking and auxiliary tasks in people with Parkinson's disease. Hum Mov Sci. 2012;31:236-246. 35. Muller SV, Jung A, Preinfalk J, Kolbe H, Ridao-Alonso M, Dengler R, Munte TF. Disturbance of "extrinsic alertness" in Huntington's disease. J Clin Exp Neuropsychol. 2002;24: 517-526. 36. De Diego-Balaguer R, Couette M, Dolbeau G, Durr A, Youssov K, Bachoud-Levi AC. Striatal degeneration impairs language learning: evidence from Huntington's disease. Brain. 2008;131:2870-2881. 37. Finke K, Schneider WX, Redel P, Dose M, Kerkhoff G, Muller HJ, Bublak P. The capacity of attention and simultaneous perception of objects: a group study of Huntington's disease patients. Neuropsychologia. 2007;45:3272-3284. 38. Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11-19. 39. Lawrence AD, Hodges JR, Rosser AE, Kershaw A, ffrench- Constant C, Rubinsztein DC, Robbins TW, Sahakian BJ. Evidence for specific cognitive deficits in preclinical Huntington's disease. Brain. 1998;121:1329-1341. 40. Ho AK, Sahakian BJ, Brown RG, Barker RA, Hodges JR, Ane M-N, Snowden J, Thompson J, Esmonde T, Gentry R, Moore JW, Bodner T. Profile of cognitive progression in early Huntington's disease. Neurology. 2003;61:1702-1706. 41. Amato MP, Ponziani G, Siracusa G, Sorbi S. Cognitive dysfunction in early-onset multiple sclerosis-a reappraisal after 10 years. Arch Neurol. 2001;58:1602-1606. 42. Achiron A, Doniger GM, Harel Y, Appleboim-Gavish N, Lavie M, Simon ES. Prolonged response times characterize cognitive performance in multiple sclerosis. Eur J Neurol. 2007;14:1102-1108. 43. Calabrese P. Neuropsychology of multiple sclerosis-an overview. J Neurol. 2006;253:I10-15. 44. Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis. Arch Neurol. 2004;61: 226-230. 45. Boringa J, Lazeron R, Reuling I, Ader H, Pfennings L, Lindeboom J, de Sonneville L, Kalkers N, Polman C. The brief repeatable battery of neuropsychological tests- normative values allow application in multiple sclerosis clinical practice. Mult Scler. 2001;7:263-267. 46. Christodoulou C, Krupp LB, Liang Z, Huang W, Melville P, Roque C, Scherl WF, Morgan T, MacAllister WS, Li L, Tudorica LA, Li X, Roche P, Peyster R. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60:1793-1798. 47. Edgar C, Jongen PJ, Sanders E, Sindic C, Goffette S, Dupuis M, Jacquerye P, Guillaume D, Reznik R, Wesnes K. Cognitive performance in relapsing remitting multiple sclerosis: a longitudinal study in daily practice using a brief computerized cognitive battery. BMC Neurol. 2011;11:68. 48. Ruff R, Hibbard K. Ruff Neurobehavioral Inventory. Odessa, FL: Psychological Assessment Resources, 2003. 49. Jamora CW, Young A, Ruff RM. Comparison of subjective cognitive complaints with neuropsychological tests in individuals with mild vs more severe traumatic brain injuries. Brain Inj. 2012;26:36-47. 50. Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, Patel MC, Counsell SJ, Sharp DJ. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449-463. 51. Cameron IG, Watanabe M, Pari G, Munoz DP. Executive impairment in Parkinson's disease: response automaticity and task switching. Neuropsychologia. 2010;48:1948-1957. 52. Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia. 2005;43:784-796. 53. Englander J, Bushnik T, Oggins J, Katznelson L. Fatigue after traumatic brain injury: association with neuroendocrine, sleep, depression and other factors. Brain Inj. 2010;24:1379-1388. 54. Feuillet L, Reuter F, Audoin B, Malikova I, Barrau K, Cherif AA, Pelletier J. Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2007;13:124-127. 55. Fielding J, Georgiou-Karistianis N, Bradshaw J, Millist L, White O. No sequence dependent modulation of the Simon 272 White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. effect in Parkinson's disease. Brain Res Cogn Brain Res. 2005;25:251-260. 56. Fielding J, Georgiou-Karistianis N, Millist L, White O. Temporal variation in the control of goal-directed visuospatial attention in basal ganglia disorders. Neurosci Res. 2006;54:57-65. 57. Corin MS, Elizan TS, Bender MB. Oculomotor function in patients with Parkinson's disease. J Neurol Sci. 1972;15:251-265. 58. Corin MS, Elizan TS, Siegel GJ. Oculomotor dysfunction in Parkinson's disease. Trans Am Neurol Assoc. 1970;95: 226-229. 59. White OB, Saint-Cyr JA, Tomlinson RD, Sharpe JA. Ocular motor deficits in Parkinson's disease. II. Control of the saccadic and smooth pursuit systems. Brain. 1983;106: 571-587. 60. Jones GM, de Jong D. Quantitative studies of eye movement in Parkinson's disease. I. Saccadic eye movements. Neurology. 1970;20:407. 61. DeJong JD, Jones GM. Akinesia, hypokinesia, and bradykinesia in the oculomotor system of patients with Parkinson's disease. Exp Neurol. 1971;32:58-68. 62. Jones GM, DeJong JD. Dynamic characteristics of saccadic eye movements in Parkinson's disease. Exp Neurol. 1971;31: 17-31. 63. Teravainen H, Calne DB. Studies of parkinsonian movement: 1. Programming and execution of eye movements. Acta Neurol Scand. 1980;62:137-148. 64. Teravainen H, Calne DB. Studies of parkinsonian movement: 2. Initiation of fast voluntary eye movement during postural disturbance. Acta Neurol Scand. 1980;62:149-157. 65. Fielding J, Georgiou-Karistianis N, White O. The role of the basal ganglia in the control of automatic visuospatial attention. J Int Neuropsychol Soc. 2006;12:657-667. 66. Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB. Inhibitory control and spatial working memory in Parkinson's disease. Mov Disord. 2007;22:1444-1450. 67. Henderson T, Georgiou-Karistianis N, White O, Millist L, Williams DR, Churchyard A, Fielding J. Inhibitory control during smooth pursuit in Parkinson's disease and Huntington's disease. Mov Disord. 2011;26:1893-1899. 68. Nakamura T, Kanayama R, Sano R, Ohki M, Kimura Y, Aoyagi M, Koike Y. Quantitative analysis of ocular movements in Parkinson's disease. Acta Otolaryngol Suppl. 1991;481:559-562. 69. Winograd-Gurvich C, Georgiou-Karistianis N, Fitzgerald PB, Millist L, White OB. Self-paced and reprogrammed saccades: differences between melancholic and non-melancholic depression. Neurosci Res. 2006; 56:253-260. 70. Lee EY, Cowan N, Vogel EK, Rolan T, Valle-Inclan F, Hackley SA. Visual working memory deficits in patients with Parkinson's disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain. 2010;133:2677-2689. 71. Lasker AG, Zee DS. Ocular motor abnormalities in Huntington's disease. Vision Res. 1997;37:3639-3645. 72. Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington's disease: slowing and dysmetria. Neurology. 1988;38:427-431. 73. Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington's disease: initiation defects and distractibility. Neurology. 1987;37:364-370. 74. Winograd-Gurvich CT, Georgiou-Karistianis N, Evans A, Millist L, Bradshaw JL, Churchyard A, Chiu E, White OB. Hypometric primary saccades and increased variability in visually-guided saccades in Huntington's disease. Neuropsychologia. 2003;41:1683-1692. 75. Blekher T, Johnson SA, Marshall BS, White K, Hui S, Weaver MS, Gray J, Yee R, Stout JC, Beristain X, Wojcieszek J, Foroud T. Saccades in presymptomatic and early stages of Huntington disease. Neurology. 2006;67:394-399. 76. Rupp J, Dzemidzic M, Blekher T, Bragulat V, West J, Jackson J, Hui S, Wojcieszek J, Saykin AJ, Kareken D, Foroud T. Abnormal error-related antisaccade activation in premanifest and early manifest Huntington disease. Neuropsychology. 2011;25:306-318. 77. Fielding J, Kilpatrick T, Millist L, Clough M, White O. Longitudinal assessment of antisaccades in patients with multiple sclerosis. PLoS One. 2012;7:e30475. 78. Fielding J, Kilpatrick T, Millist L, White O. Antisaccade performance in patients with multiple sclerosis. Cortex. 2009;45:900-903. 79. Fielding J, Kilpatrick T, Millist L, White O. Control of visually guided saccades in multiple sclerosis: disruption to higher-order processes. Neuropsychologia. 2009;47:1647-1653. 80. Fielding J, Kilpatrick T, Millist L, White O. Multiple sclerosis: cognition and saccadic eye movements. J Neurol Sci. 2009;277:32-36. 81. Pearson BC, Armitage KR, Horner CW, Carpenter RH. Saccadometry: the possible application of latency distribution measurement for monitoring concussion. Br J Sports Med. 2007;41:610-612. White and Fielding: J Neuro-Ophthalmol 2012; 32: 266-273 273 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |