| OCR Text |
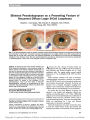
Show Bilateral Pseudohypopyon as a Presenting Feature of Recurrent Diffuse Large B-Cell Lymphoma Stephen J. Dorrepaal, MD, Edward A. Margolin, MD, FRCSC, Chen Wang, MD, PhD, FRCPC Abstract: A 55-year-old man with Gaucher disease and B-cell lymphoma developed a white meniscus along the inferior portion of the anterior chamber of both eyes. In one eye, the meniscus was also temporal, reflecting the fact that he had just been lying on his left side. Aspiration of aqueous fluid confirmed that the meniscus was made up of lymphoma cells, indicating that it was a pseudohy-popyon. (A true hypopyon is made up of reactive white blood cells.) Despite intensive chemotherapy, the patient expired within 14 weeks of the discovery of the pseudohypopyon. This is the first report of binocular pseudohypopyon confirmed as lymphomatous by flow cytometric immunophenotyping analysis in a patient with diffuse large B-cell lymphoma. Journal of Neuro-Ophthalmology 2010;30:67-69 doi: 10.1097/WNO.0b013e3181ce2c98 2010 by North American Neuro-Ophthalmology Society A55-year man with a history of Gaucher disease was diagnosed with stage 4 diffuse large B-cell lymphoma after presenting with exertional dyspnea due to a large mediastinal mass. The staging workup revealed atypical lymphocytes in the cerebrospinal fluid (CSF) and bone marrow. Initial treatment consisted of 8 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednis-olone (R-CHOP) and 10 cycles of intrathecal methotrexate and cytarabine chemotherapy. Afterward, his disease was deemed to be in remission. Subsequent restaging inves-tigations 2 and 4 months later revealed interval decrease in residual node size, and radiotherapy was not performed. Six months after the initial diagnosis the patient was admitted to the hospital with progressive bilateral arm weakness and shooting pains in all 4 limbs. Differential diagnosis at the time included lymphomatous infiltration of peripheral nerves and roots, vincristine toxicity, and an acute demyelinating disorder. Intravenous immunoglobulin (IVIg) was administered for 2 days with minimal improvement in the patient's symptoms. During the hospital stay, the patient noticed that vision in both eyes would become cloudy upon forward and backward neck flexion. Full resolution of visual symptoms FIG. 1. Slit lamp photography shows an anterior chamber cream-colored meniscus inferiorly in the right eye and inferiorly and temporally in the left eye. The patient had been photographed in the upright position hours after awakening from having slept on his left side. Hours later, the temporal component of the meniscus in the left eye had disappeared. Aspiration of the meniscus of the right eye revealed lymphoma cells (inset), indicating that the meniscus represented a ‘‘pseudohypopyon'' rather than a true hypopyon, which is composed of pus. Departments of Ophthalmology and Visual Sciences (SJD, EAM) and Pathology and Laboratory Medicine (CW), University of Toronto, Toronto, Ontario, Canada. Address correspondence to Edward A. Margolin, MD, FRCSC, University of Toronto, Department of Ophthalmology and Visual Sciences, Neuro-Ophthalmology, 600 University Avenue, Suite 409, Toronto, ON M5G 1X5, Canada; E-mail: edmargolin@gmail.com Dorrepaal et al: J Neuro-Ophthalmol 2010; 30: 67-69 67 Photo Essay occurred rapidly after resuming an upright posture. He had no eye pain or photophobia. Visual acuity was 20/20 in both eyes, pupillary responses were normal, and intraocular pressures were within normal ranges. On biomicroscopic examination, both irides appeared thickened and ‘‘boggy'' and a 3+ fine cellular reaction was visible in both anterior chambers. There was an anterior chamber cream-colored meniscus inferiorly in the right eye and inferiorly and temporally in the left eye (Fig. 1). The explanation for the temporal component of the meniscus was that our examination was performed in the morning shortly after the patient had slept on his left side. When he was reexamined several hours later, the temporal component of the left eye meniscus had disappeared. Anterior chamber paracentesis of the right eye removed 0.15 mL of aqueous, which revealed medium-large atypical lymphoid cells (Fig. 1, inset). Flow cytometry revealed a clonal population of B cells with the phenotype CD19+ (99.3%), CD5, CD23, CD10, FMC7, and lambda light chain restriction (98.5%). The phenotypic findings were consistent with B-cell lymphoma, indicating that the anterior chamber was a pseudohypopyon rather than a true hypopyon made up of reactive white blood cells. Cerebrospinal fluid, obtained by lumbar puncture that day, also revealed malignant lymphocytes. The patient was treated with high-dose oral dexameth-asone and hourly topical prednisolone drops. The hypopyon decreased in size initially but recurred after dexamethasone taper was attempted. Two weeks later, the patient noticed binocular oblique diplopia. Examination disclosed a partial left third cranial nerve palsy. MRI of the brain and orbits did not reveal any abnormalities. CSF analysis again revealed cellular atypia and a monoclonal B-cell population consistent with lymphoma. He received fractionated X-irradiation to the eyes, orbits, and total brain, with a total dose of 20 Gy in 5 fractions over 5 days. Over the following month, the third cranial nerve palsy and hypopyon resolved. Two months later, the patient was neuro-ophthalmo-logically normal. However, clonal B lymphocytes were found in a peripheral blood sample, and treatment with cisplatin, dexamethasone, and gemcitabine was started. He also received red blood cell and platelet transfusions over the following week for anemia and thrombocytopenia. One month later, he died, only 14 weeks after the discovery of the hypopyon. ‘‘Pseudohypopyon'' refers to an accumulation of neo-plastic cells in the anterior chamber, whereas a true hypopyon is made up of reactive white blood cells. Pseudohypopyon may be seen with a variety of intraocular tumors, including lymphoma, leukemia, and retinoblas-toma, and may persist despite corticosteroid treatment (1,2). Typically a pseudohypopyon will settle inferiorly, but layers may settle at different orientations depending on positioning, as occurred in our patient. Our patient had Gaucher disease, which has a strong association with hematologic malignancy, including diffuse large B-cell lymphoma (3-5). Lymphoma is one of the classic uveitis masquerade syndromes, accounting for up to 1.6% of all patients with uveitis seen in one study at a tertiary ophthalmologic center (6). In an autopsy study of patients with lymphoma at the time of death, 6.7% of eyes were found to have uveal infiltrates (7). Lymphomatous involvement of the anterior chamber may arise either via hematogenous spread or a recurrence of preexisting anterior chamber cells that were suppressed but not eradicated by previous chemotherapy (8). There are two distinct types of intraocular lymphoma: primary central nervous system (CNS) lymphoma (including primary intraocular lymphoma), and lymphoma that arises outside the CNS andmetastasizes hematogenously, usually to the uveal tract. Ocular involvement is much less prevalent in lymphoma arising outside the CNS; isolated pseudohypopy-on in such cases is rare (9). There are a few reports of patients in whompseudohypopyon was among the presenting signs of a systemic lymphoma (2,10-15). Pseudohypopyon has also been reported as heralding a relapse among patients with systemic lymphoma in remission (10,16-19). Bilateral lymphomatous pseudohypopyon is exceedingly rare. One case was reported in a patient with known cutaneous natural killer cell lymphoma and another in a patient with AIDS and non-Hodgkin lymphoma (18,20). Both of these presentations occurred late in the course of the disease, within 2 months of death. This is the only report of bilateral pseudohypopyon in a patient with diffuse large B-cell lymphoma confirmed by flow cytometric immunophe-notyping analysis. This case illustrates the importance of suspecting masquerade syndrome in patients with an atypical hypopyon and a history of previous hematologic malig-nancy. Diagnosis of pseudohypopyon may be confirmed with flow cytometric immunophenotyping of an anterior chamber aspirate. Anterior chamber involvement may be a presenting feature of recurrent lymphoma even in the absence of negative imaging and CSF examination results. Bilateral lymphomatous pseudohypopyon has only been reported in advanced disease and portends a poor prognosis. ACKNOWLEDGMENT We thank Alan Connor, Ocular Oncology Clinic, Princess Margaret Hospital, Toronto, for taking the photograph featured in Figure 1. The patient featured in Figure 1 is deceased. Permission for the publication of this figure was provided by his wife. REFERENCES 1. Ramsay A, Lightman S. Hypopyon uveitis. Surv Ophthalmol 2001;46:1-18. 2. Goldey SH, Stern GA, Oblon DJ, et al. Immunophenotypic characterization of an unusual T-cell lymphoma presenting Photo Essay 68 Dorrepaal et al: J Neuro-Ophthalmol 2010; 30: 67-69 as anterior uveitis. A clinicopathologic case report. Arch Ophthalmol 1989;107:1349-53. 3. Shiran A, Brenner B, Laor A, et al. Increased risk of cancer in patients with Gaucher disease. Cancer 1993;72:219-24. 4. Perales M, Cervantes F, Cobo F, et al. Non-Hodgkin's lymphoma associated with Gaucher's disease. Leuk Lymphoma 1998;31:609-12. 5. Brody JD, Advani R, Shin LK, et al. Splenic diffuse large B-cell lymphoma in a patient with type 1 Gaucher disease: diagnostic and therapeutic challenges. Ann Hematol 2006;85:817-20. 6. Rothova A, Ooijman F, Kerkhoff F, et al. Uveitis masquerade syndromes. Ophthalmology 2001;108:386-99. 7. Nelson CC, Hertzberg BS, Klintworth GK. A histopathologic study of 716 unselected eyes in patients with cancer at the time of death. Am J Ophthalmol 1983;95:788-93. 8. Ninane J, Taylor D, Day S. The eye as a sanctuary in acute lymphoblastic leukaemia. Lancet 1980;1:452-63. 9. Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol 2002;13:411-8. 10. O'Keefe JS, Sippy BD, Martin DF, et al. Anterior chamber infiltrates associated with systemic lymphoma: report of two cases and review of the literature. Ophthalmology 2002;109:253-7. 11. Weisenthal R, Frayer WC, Nichols CW, et al. Bilateral ocular disease as the initial presentation of malignant lymphoma. Br J Ophthalmol 1988;72:248-52. 12. Espana-Gregori E, Hernandez M, Menezo-Rozalen JL, et al. Metastatic anterior chamber non-Hodgkin lymphoma in a patient with acquired immunodeficiency syndrome. Am J Ophthalmol 1997;124:243-5. 13. Soylu M, O¨ zcan AA, Okay O, et al. Non-Hodgkin lymphoma presenting with uveitis occurring after blunt trauma. Pediatr Hematol Oncol 2005;22:53-7. 14. Lobo A, Larkin G, Clark BJ, et al. Pseudo-hypopyon as the presenting feature in B-cell and T-cell intraocular lymphoma. Clin Exp Ophthalmol 2009;31: 155-8. 15. Gu¨ndu¨z E, Korkmaz C, Kasifoglu T, et al. Unusual presentation of non-Hodgkin lymphoma: polyarthritis and uveitis mimicking a rheumatologic disease. Rheumatol Int 2008;28:613-24. 16. Verity DH, Graham EM, Carr R, et al. Hypopyon uveitis and iris nodules in non-Hodgkin's lymphoma: ocular relapse during systemic remission. Clin Oncol 2000;12: 292-4. 17. Papaliodis GN, Montezuma SR. Pseudo-hypopyon and the presenting feature of recurrent b-cell lymphoma. Ocul Immunol Inflamm 2008;16:121-32. 18. Hon C, Kwok AK, Shek TW, et al. Unusual locations of involvement by malignancies, case 4: bilateral hypopyon heralding CNS relapse of cutaneous natural killer cell lymphoma. J Clin Oncol 2003;21: 3373-84. 19. Corriveau C, Easterbrook M, Payne D. Lymphoma simulating uveitis (masquerade syndrome). Can J Ophthalmol 1986;21:144-9. 20. Jan NA, Einzig AI, Suhrland MJ, et al. Non-Hodgkin lymphoma in acquired immunodeficiency syndrome manifesting a bilateral hypopyon. Am J Clin Oncol 1999;22: 82-93. Photo Essay Dorrepaal et al: J Neuro-Ophthalmol 2010; 30: 67-69 69 |