| OCR Text |
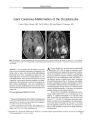
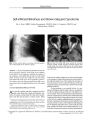
Show ORIGINAL CONTRIBUTION Persistent Severe Visual and Electroretinographic Abnormalities After Intravenous Cisplatin Therapy Bradley J. Katz, MD, PhD, John H. Ward, MD, Kathleen B. Digre, MD, DonnellJ. Creel, PhD, and Nick Mamalis, MD Abstract: A 55- year- old man inadvertently received four times the intended dose of intravenous cisplatin as part of a chemotherapeutic salvage regimen for non- Hodgkin lymphoma. Immediately after treatment, he developed bilateral irreversible visual loss. Visual acuity was 20/ 300 in OU and visual fields showed central scotomas bilaterally. Although the fundus examination findings were normal, an electrore-tinogram showed markedly reduced a- wave amplitudes and absent b- waves. At autopsy 8 months later, photoreceptors appeared normal. Splitting of the outer plexiform layer was present, consistent with loss of the ERG b- wave. This is the first reported case ofpersistent visual loss from intravenous cisplatin toxicity and the first case to describe ocular histopathologic findings. ( JNeuro- Ophthalmol 2003; 23: 132- 135) any cases of intermediate- and high- grade non- Hodgkin lymphomas ( NHL) respond to combination chemotherapy. For recurrent disease, salvage regimens offer an opportunity for a second remission but little chance for long- term control. Salvage regimens are often complicated and may involve continuous infusions of some agents. These regimens use maximally tolerated doses, so there is little margin for error. Well- publicized examples of dosage errors have been reported in the lay press. We report a case in which a patient received cisplatin as part of a salvage regimen for NHL. Because of a dosage miscalculation, he received four times the planned dose of cisplatin before referral to our institution. Consequently, he Department of Ophthalmology and Visual Sciences and the John A Moran Eye Center ( BJK, KBD, DJC, NM), the Department of Neurology ( BJK, KBD), and the Oncology Division, Department of Internal Medicine and the Huntsman Cancer Institute ( JHW), University of Utah Health Sciences Center, Salt Lake City, Utah. Address correspondence to Bradley J. Katz, MD, PhD, John A Moran Eye Center, University of Utah, 50 North Medical Drive, Salt Lake City, UT 84132, USA; E- mail: bradley. katz@ hsc. utah. edu Supported in part by an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness, Inc., New York, New York. suffered severe, permanent vision loss due to cisplatin-induced retinal toxicity. We report the first case of persistent visual loss in this setting and document the retinal abnormalities found on autopsy. CASE REPORT A 55- year- old man developed night sweats, weight loss, and a nonproductive cough. Cervical and intraabdominal lymphadenopathy were discovered and a biopsy revealed a diffuse large cell lymphoma, B- cell phenotype. He received six cycles of CHOP ( cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy and achieved a complete remission. Four months later, the patient developed bilateral axillary and retroperitoneal lymphadenopathy. He received ESHAP ( cisplatin, etoposide, high- dose cytarabine, and methylprednisolone) chemotherapy. The planned regimen included a 4- day continuous infusion of cisplatin at a dose of 25 mg/ m2 daily for a total cisplatin dose of 100 mg/ m2 over 96 hours. The actual delivered cisplatin dose was 100 mg/ m2 daily for 4 days, or 400 mg/ m2 total dose. Two days after the conclusion of chemotherapy, he was admitted to the hospital with anorexia, nausea, tinnitus, decreased vision, and loss of color perception. He was found to have acute renal failure, visual impairment, severe hepatotoxicity, and pancytopenia. Following a 1- month hospitalization, he was referred to the University of Utah for further care. Best- corrected visual acuity was 20/ 300 in OU and the patient was unable to identify any of the Hardy- Rand- Rittler color plates. He had no congenital color vision deficit by history. Pupils were normally reactive and there was no afferent pupillary defect. He had a central scotoma in OU by confrontation and extraocular motility was full. Anterior segments were quiet with mild nuclear sclerosis and posterior subcapsular cataract. Intraocular pressures were normal. Fundus examination was normal except for a choroidal nevus in the temporal periphery of the OD. There was no optic nerve edema or pallor and no visible abnormalities of the macular retinal pigment epithelium. Goldmann visual field testing revealed mild, generalized constriction and Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 132 J Neuro- Ophthalmol, Vol. 23, No. 2, 2003 CISPLATINRETINAL TOXICITY JNeuro- Ophthalmol, Vol. 23, No. 2, 2003 central scotomas in OU, as well as an inferior arcuate scotoma in the OD. His physical examination revealed no evidence of lymphoma. He had a dense, bilateral, stocking- glove neuropathy to pinprick and vibration and his deep tendon reflexes were diminished. An electroretinogram ( ERG) ( 1) revealed diminished a- wave amplitudes and complete loss of the b- wave ( Fig. 1). Magnetic resonance imaging of the brain showed no lymphomatous involvement of the brain, meninges, or orbits, or any other pathologic lesion of the central visual pathways. Six months later, the patient developed progressive cervical and supraclavicular adenopathy. He received cyclophosphamide, etoposide, procarbazine, and prednisone. His adenopathy promptly resolved but his neurologic deficits remained unchanged. Two months later, he died of clostridial sepsis. At 6 weeks prior to death, his visual acuity was unchanged at 20/ 300 in OU. Both globes were obtained at autopsy and examined by an ocular pathologist ( NM). On gross pathologic examination, the globes appeared completely normal. On microscopic examination ( Fig 2), the retina showed artefactual detachment secondary to processing and cutting. The ganglion cell layer was mildly attenuated temporally, being approximately three to four cell layers thick. The inner nuclear layer also showed some irregularity with a decrease in cell thickness from approximately four or five cell layers to approximately two cell layers in some sections. The outer plexiform layer showed a small amount of splitting. The outer nuclear layer and photoreceptor outer segments were normal. Sections of the optic nerve were stained for both axons and myelin, and all sections appeared normal. In the general autopsy examination, there were no abnormalities of the central visual pathways. DISCUSSION Cisplatin ( cw- dichlorodiammine platinum [ II]) is used in the treatment of both systemic and central nervous system malignancies and is thought to exert its biologic effect by binding directly to DNA. The major toxic effects of cisplatin are nephrotoxicity, ototoxicity, and a peripheral neuropathy. Irreversible vision loss may result from effects on the retina, optic nerve, or cortical visual pathways ( 2). Retinal toxicity has been reported after therapeutic, high- dose intravenous administration of cisplatin and can be associated with pigmentary changes in the macula. Wilding et al. ( 3) studied 13 patients receiving high- dose intravenous cisplatin ( 200 mg/ m2 in five divided daily doses over two to four cycles). Eight patients reported blurred vision and three of these patients also described altered color perception. Nine of 11 patients studied had ERG abnormalities. During the follow- up period, symptoms of blurred vision gradually dissipated, although three patients continued to note altered color perception, and abnormalities in the ERG persisted. Unfortunately, data regarding visual acuity were not reported, although the authors implied that no patients had persistent, significant visual impairments. By contrast, intracarotid infusion of cisplatin, occasionally used in the treatment of cerebral malignancies, can produce significant permanent visual impairment. Five series of patients experiencing visual loss associated with the intracarotid infusion of cisplatin have been reported ( 4- 8). The pathophysiology of the visual loss was not completely W w * , * W ' ^ o ^ ^ FIG. 1 . Electroretinogram obtained from the patient's OD. The traces in the left column were obtained from the patient. Traces in the right column were recorded from a normal patient, for comparison. Patient responses to the photopic white flash, the scotopic blue flash, and the 30 Hz red flicker are all nonrecord-able; a small a- wave, but no b- wave, was recorded in response to a scotopic white flash. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 133 JNeuro- Ophthalmol, Vol. 23, No. 2, 2003 Katz et al. * u FIG. 2. Histopathologic specimen of the patient's OS obtained at autopsy. Hematoxylin and eosin stain. A: At low magnification, artefactual retinal detachment is seen with a grossly normal retinal pigment epithelium and choroid ( original magnification 25 x ) . B: At higher magnification, splitting of the outer plexiform layer is seen ( original magnification 100X). C: At the highest magnification, the photoreceptor outer segments and inner nuclear layer appear normal. There is splitting of the outer plexiform layer. The outer nuclear layer and ganglion cell layers are mildly attenuated ( original magnification 200 x ) . Bodian stain of the optic nerve showed normal nerve fibers and Luxol fast blue stain of the optic nerve revealed normal myelin ( not shown). investigated in every case, but it appears that visual loss can occur from effects on both the retina and the optic nerve. Although visual loss was typically ipsilateral to the placement of the infusion catheter, there are some patients who experienced bilateral visual impairment. In two series in which intracarotid infusion of cisplatin was accompanied by intravenous administration of carmustine ( BCNU), some patients developed vision loss and an unusual pigmentary maculopathy ( 9,10). Because of its toxicity, intracarotid cisplatin is no longer in common use. Marmor ( 11) reported a single case of vision loss associated with inadvertent intravenous cisplatin overdose. A 68- year- old woman being treated for ovarian cancer suffered loss of visual acuity after receiving 480 mg of cisplatin, roughly seven times the usual dose. Her acuity returned to normal, but she had persistent ERG abnormalities, including reduced b- waves and oscillatory potentials, and a pigmentary maculopathy. The only previous report of cisplatin- associated vision loss with autopsy material ( 12) involved a 3 1/ 2- year-old girl treated with cisplatin for a sacrococcygeal teratoma. The patient initially received five cycles of cisplatin, 120 mg/ m2. In the course of treatment, she developed bilateral optic disc edema, bilateral hearing loss, renal toxicity, loss of muscle stretch reflexes, and a right foot drop. At autopsy, both optic nerve heads were swollen, but there was no comment about histopathologic examination of the retina or about axonal loss or alterations in myelination within the optic nerve. There was also no mention of visual acuity or funduscopic examination of the retina. Although the ERG a- wave in our patient was substantially attenuated, the photoreceptors appeared normal on histopathologic examination. One could speculate that cisplatin is toxic to photoreceptor function, but that it does not always cause morphologic changes in the photoreceptors. Alternatively, because only 1 year had elapsed between the administration of cisplatin and the patient's death, it is possible that photoreceptor degeneration would have occurred if given sufficient time. The splitting observed in the outer plexiform layer may explain the loss of the b- wave and the preservation of a small a- wave in both our patient and the patient described by Marmor ( 11). Splitting of the outer plexiform layer would result in the loss of transmission of signals between the photoreceptors and the inner retina. This alteration in the outer plexiform layer may also explain attenuation of the b- wave observed in some patients receiv- Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 134 © 2003 Lippincott Williams & Wilkins CISPLATINRETINAL TOXICITY JNeuro- Ophthalmol, Vol. 23, No. 2, 2003 ing intra- arterial cisplatin and carmustine ( 7). Other substances that are associated with selective or predominant decrease in b- wave amplitude include iron, methanol, quinine, vincristine, canthaxanthin, glycine, and vigabatrin ( 13). Loss of the b- wave has also been reported with an occupational exposure to the veterinary anesthetic ethyl- w-aminobenzoic acid methane sulfonate ( MS- 222) ( 14). Paraneoplastic antibodies, as in carcinoma- associated retinopathy ( 15) and melanoma- associated retinopathy ( 16), have been shown to cause severe, irreversible vision loss along with attenuation of the ERG. Our patient was not tested for the presence of antiretinal antibodies, which we considered an unlikely cause of his vision loss. First, these retinopathies are almost always associated with solid tumors and there is only one report in the literature of a slowly progressive, lymphoma- associated retinopathy ( 17). Second, patients with vision loss associated with paraneoplastic retinopathies usually describe photopsias and progressive loss of visual acuity, in contrast to our patient's abrupt, bilateral, simultaneous loss of vision. Although retinal toxicity associated with both intravenous and intra- arterial cisplatin has been previously reported, this is the first case in which intravenous administration of cisplatin has been associated with severe retinal toxicity, enough to cause irreversible loss of vision. The fact that irreversible vision loss, previously associated only with intracarotid administration of cisplatin, can also occur with intravenous administration lends further support to the hypothesis that the toxic effects of cisplatin on the retina are dose- dependent ( 2). This case is also the first case in which histologic sections of retina have been obtained. The splitting observed in the outer plexiform layer may explain the loss of the b- wave documented in our patient, as well as in other patients receiving large amounts of cisplatin. The mechanism that causes this observed splitting is unknown. REFERENCES 1. Richards SC, Creel DJ. Pattern dystrophy and retinitis pigmentosa caused by a peripherin/ RDS mutation. Retina 1995; 15: 68- 72. 2. Fraunfelder FT, Fraunfelder FW. Drug- Induced Ocular Side Effects, edn 5. Boston: Butterworth Heinemann, 2001: 446- 450. 3. Wilding G, Caruso R, Lawrence TS, et al. Retinal toxicity after high- dose cisplatin therapy. J Clin Oncol 1985; 3: 1683- 9. 4. Stewart DJ, Wallace S, FeunL, etal. A phase I study of intracarotid artery infusion of cis- diamminedichloroplatinum( II) in patients with recurrent malignant intracerebral tumors. Cancer Res 1982; 42: 2059- 62. 5. Feun LG, Wallace S, Stewart DJ, et al. Intracarotid infusion of cis-diamminedichloroplatinum in the treatment of recurrent malignant brain tumors. Cancer 1984; 54: 794- 9. 6. Tang R, Marroquin G, Feun LG. Ocular toxicity and intracarotid infusion of cisplatin. Cancer Bull 1985; 37: 35- 37. 7. Kupersmith MJ, Frohman LP, Choi IS, et al. Visual system toxicity following intra- arterial chemotherapy. Neurology 1988; 38: 284- 9. 8. Maiese K, Walker RW, Gargan R, et al. Intra- arterial cisplatin- as-sociated optic and otic toxicity. Arch Neurol 1992; 49: 83- 6. 9. Miller DF, Bay JW, Lederman RJ, et al. Ocular and orbital toxicity following intracarotid injection of BCNU ( carmustine) and cisplati-num for malignant gliomas. Ophthalmology 1985; 92: 402- 6. 10. Kupersmith MJ, Seiple WH, Holopigian K, et al. Maculopathy caused by intra- arterially administered cisplatin and intravenously administered carmustine. Am J Ophthalmol 1992; 113: 435- 8. 11. Marmor MF. Negative- type electroretinogram from cisplatin toxicity. Doc Ophthalmol 1993; 84: 237^ 16. 12. Walsh TJ, Clark AW, Parhad IM, et al. Neurotoxic effects of cisplatin therapy. Arch Neurol 1982; 39: 719- 20. 13. Fishman GA, Birch DG, Holder GE, et al. Electrophysiologic Testing in Disorders of the Retina, Optic Nerve, and Visual Pathway, edn 2. San Francisco: Foundation of the American Academy of Ophthalmology, 2001: 88- 99. 14. Bernstein PS, Digre KB, Creel DJ. Retinal toxicity associated with occupational exposure to the fish anesthetic MS- 222. Am J Ophthalmol 1997; 124: 843^ t. 15. Thirkill CE, Roth AM, Keltner JL. Paraneoplastic retinopathy syndrome. Ophthalmology 1993; 100: 147. 16. Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma- associated retinopathy syndrome: eleven new cases and a review of 51 previously published cases. J Neurooph-thalmol 2001; 21: 173- 87. 17. To KW, Thirkill CE, Jakobeic FA, et al. Lymphoma- associated retinopathy. Ophthalmology 2002; 109: 2149- 53. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 135 |