| OCR Text |
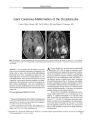
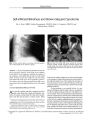
Show ORIGINAL CONTRIBUTION Late Ipsilateral Recurrence of Ischemic Optic Neuropathy in Giant Cell Arteritis Nancy Kim, PhD, Jonathan D. Trobe, MD, Andrew Flint, MD, and Gary Keoleian, MD Abstract: A patient with arteriosclerosis, diabetes melli-tus, and giant cell arteritis ( GCA) treated continuously with low- dose prednisone developed anterior ischemic optic neuropathy ( AION) at 5 and 13 months after clinical diagnosis of GCA. At the time of late recurrent AION, there were no systemic symptoms or elevations in acute phase reactants to signal active arteritis, yet temporal artery biopsy disclosed dramatic inflammation, forcing the presumption that the infarct was arteritic. Recurrent systemic symptoms and elevation of acute phase reactants are not reliable warning signs of reactivated GCA. In patients at high risk for corticosteroid complications, late biopsy may be a reasonable guide to corticosteroid weaning. ( JNeuro- Ophthalmol 2003; 23: 122- 126) Giant cell arteritis ( GCA) is a systemic, necrotizing vasculitis of the elderly primarily affecting large and medium size cranial arteries originating from the aorta ( 1- 3). It presents with fever, myalgia, malaise, weight loss, headache, temporal or scalp tenderness, jaw claudication, and acute, painless visual loss ( 4), the most serious complication. Typically, the cause of visual loss is anterior ischemic neuropathy ( AION) secondary to inflammatory occlusion of the short posterior ciliary arteries supplying the laminar and retrolaminar regions of the optic disk ( 4- 7). The accepted treatment of GCA is intravenous meth-ylprednisolone at 1 to 2 g/ d for 3 to 5 days or oral prednisone at 1 to 2 mg/ kg/ d for the initial 2 to 4 weeks pending clinical improvement and normalization of acute phase reactants ( erythrocyte sedimentation rate [ ESR] and/ or C-reactive protein [ CRP]), followed by a slow prednisone taper to a baseline dose that suppresses reactivation of symptoms and elevation of acute phase reactants ( 8- 10). Departments of Ophthalmology ( NK, JDT), Neurology ( JDT), and Pathology ( AF), University of Michigan Medical School, Ann Arbor, Michigan, and the Michigan Eye Institute ( GK), Flint, Michigan. Address correspondence to Jonathan D. Trobe, MD, Kellogg Eye Center, 1000 Wall Street, Ann Arbor, MI 48105, USA; E- mail: jdtrobe@ umich. edu Once the systemic symptoms and acute phase reactants have normalized, future episodes of AION are relatively uncommon ( 9,11- 13). Late recurrent visual loss in the ipsilateral eye is even rarer ( 9,11- 13). Recurrent symptoms generally include malaise, weight loss, and myalgia, headache, or scalp tenderness ( 9,14- 18). We present a case in which prednisone therapy failed to prevent AION at 5 and 13 months after initial diagnosis without any recrudescence in systemic symptoms or elevation in acute phase reactants. Temporal artery biopsy, performed at the time of the recurrent ipsilateral AION, was floridly positive. CASE REPORT In October 2000, an 83- year- old man presented to his primary care physician with a several day history of severe headache, arthralgia, and myalgia. He had longstanding hypertension, noninsulin- dependent diabetes mellitus, and hyperlipidemia. Thirty years earlier, he had experienced sudden severe and permanent visual loss in the OS that had been attributed to a retinal artery occlusion. He had no abnormalities in the OD. Physical examination was normal. Westergren ESR was 101 mm/ h and CRP was 15.4 mg/ dl. MRI and MRA of the brain showed remote, focal ischemic injury but no vessel abnormalities. Based on the symptoms and marked elevation in acute phase reactants, a presumptive diagnosis of GCA was made and the patient was begun on prednisone 60 mg/ d ( Fig. 1). No temporal artery biopsy was performed at this time. Within a few days, the patient's symptoms had completely resolved. Over the next 6 weeks, prednisone was tapered to 20 mg/ d with good symptom control and reduction of the ESR to 10 mm/ h. Because of the patient's ample arteriosclerotic risk factors, he was considered highly vulnerable to corticosteroid complications. Therefore, within 2 months after clinical diagnosis of GCA, the prednisone dose had been further reduced to 20 mg every other day. The patient remained asymptomatic on this maintenance dose until 5 months after diagnosis, in early March 2001, when he suddenly developed acute visual loss in the OD. At that time, systemic medications also included digoxin 0.25 mg/ d, amlodipine besylate 5 mg/ d, metoprolol 50 mg/ d, furosemide 40 mg/ d, metformin hydrochloride, Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 122 J Neuro- Ophthalmol, Vol. 23, No. 2, 2003 ISCHEMIC OPTIC NEUROPATHY JNeuro- Ophthalmol, Vol 23, No. 2, 2003 . Clinical diagnosis i olGCAfno bopsy) • Erythrocyte sedimentation rate Prednisone dose Acme visual loss OD second e pi soda 1 Temporal artery * / biopsy with * florid arteritis " I FIG. 1. Our patient's clinical course. and simvastatin 20 mg/ d without recent dosage changes. Blood pressure was 170/ 85, pulse 60 and regular, and there were no cardiac or cervical auscultatory abnormalities. Ophthalmologic examination disclosed visual acuities of 20/ 30 OD, 20/ 200 OS. No afferent pupillary defect was present in the OD. Ophthalmoscopy revealed a swollen superior optic disk OD and a corresponding inferior altitu-dinal visual field defect ( Fig. 2). The left fundus appeared unchanged. ESR and CRP at this time were 27 mm/ h and 0.5 mg/ dl, considered within the normal range. He received a presumptive diagnosis of arteritic AION and the prednisone dose was increased to 60 mg/ d. The prednisone was gradually tapered over the next 6 months to 10 mg/ d as the ESR and CRP remained normal ( Fig 1). In late November 2001, 13 months after his original diagnosis and 8 months after the development of AION in the OD, the patient developed recurrent acute visual loss in that eye. He denied any symptoms compatible with systemic hypotension, and no major drop in blood pressure had been recorded. Visual acuity had fallen from 20/ 25 to 20/ 50 OD and an afferent pupil defect was now apparent. The visual field disclosed extension of the defect superiorly. Swelling of the optic disk was again observed, this time in the inferior segment ( Fig. 3). Prednisone was increased to 60 mg/ d. A temporal artery biopsy obtained a week later revealed dramatic inflammatory infiltration of the vessel with giant cells, histiocytes, and lymphocytes, as well as severe destruction of the internal elastica ( Fig. 4). In June 2002, 7 months after the second attack of arteritic AION OD, examination disclosed stable visual acuities of OD 20/ 50, OS 20/ 200 with a pale disc OD. The patient had been slowly tapered to a dose of prednisone 10 mg/ d. ESR was 2 mm/ h. DISCUSSION Despite continuous low- dose maintenance prednisone therapy, our patient developed two attacks of AION FIG. 2. A: First attack of ischemic optic neuropathy OD. Funduscopy shows edema of superior optic disk. B: Visual field shows the inferior nerve fiber bundle defect. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 123 JNeuro- Ophthalmol, Vol. 23, No. 2, 2003 Kim et al. FIG. 3. Second attack of ischemic optic neuropathy OD. A: Funduscopy shows edema of the inferior optic disk. B: The visual field shows extension of the defect to superior quadrants. in the same eye at 5 and 13 months after initial clinical diagnosis of GCA. Because of the floridly positive biopsy for GCA at 13 months, we presume that each attack represented arteritic rather than nonarteritic vaso- occlusion. Yet there was no recurrence of systemic symptoms or rise in acute phase reactants to signal a flare- up. A normal ESR at presentation does not preclude the diagnosis of GCA ( 19- 21). In a retrospective series, Ellis and Ralston ( 22) found that 18 ( 23%) of 80 patients in a population treated for GCA had presented with an ESR within the normal range. Monitoring acute phase reactants may also be an ineffectual means of detecting GCA recrudescence. A long- term study of 77 GCA patients who had an initial ESR higher than 30 mm/ hr ( 23) found that clinical relapses late in the course of treatment were associated with an elevated ESR in only 43% of cases and an elevation of CRP in only 35% of cases. While there have been a number of investigations of other inflammatory mediators as potential markers of GCA disease activity ( 24- 26), no clearly reliable alternatives have emerged. • K- ^ a- .- * > ' ' i & h% Yz ***** ^-?* r^ **..* -•' K..-:&"" T-FIG. 4. Temporal artery biopsy. The media and adventitia contain numerous lymphocytes, histiocytes, and plasma cells. Multinucleated giant cells are present at the border between the fibrotic and thickened intima and the media ( hematoxylin and eosin). Inset: Higher power shows multinucleated giant cells. Our patient's visual relapse occurred not only without a rise in acute phase reactants, but also without warning symptoms. Most relapses during long- term treatment of GCA consist of fever, malaise, myalgias, jaw claudication, scalp tenderness, or temporal headache ( 9,14- 18). Late visual loss is uncommon and prior reports of this phenomenon do not provide adequate documentation regarding concurrent corticosteroid doses, systemic symptoms, or acute phase reactants ( 9,11- 13,27). In a series of 45 GCA patients, Liu et al ( 11) described six in whom AION occurred at 1 month to 6 years after presentation and the initiation of corticosteroid treatment. In three patients, prednisone doses were 10 to 40 mg/ d at the time of visual loss. In a fourth patient, prednisone was 5 mg taken on alternate days. In the fifth patient, the prednisone had been discontinued 1 week before the visual loss; no dose information is available for the sixth patient. ESR was elevated in only three patients at the time of late visual loss. There is no information regarding systemic symptoms accompanying these events. Late ipsilateral recurrence of AION in patients with GCA is very rare. Calamia and Hunder ( 12) described a patient who experienced recurrent ipsilateral visual loss 5 months after initiation of treatment with prednisone 25 mg/ d. At the time of the initial episode of visual loss, ophthalmologic examination revealed an afferent pupillary defect OD, a visual acuity of 14/ 21, and slight blurring of the right nasal optic disc margin. At the time of the recurrent ipsilateral visual loss, visual acuity had worsened to finger counting OD, but no further clinical details are provided. However, visual acuity returned to baseline OD with an increase in the prednisone dose to 60 mg/ d. In their review of GCA cases, Liu et al ( 11) reported recurrent AION in the same eye in two patients ( patients # 8, # 10) at 8 months and 14 months after initiation of continuous prednisone therapy. Patient # 8 was taking prednisone 5 mg/ d at the time of visual recurrence. However, other information regarding the presence or absence of recurrent Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 124 © 2003 Lippincott Williams & Wilkins ISCHEMIC OPTIC NEUROPATHY JNeuro- Ophthalmol, Vol. 23, No. 2, 2003 symptoms, laboratory findings, or corticosteroid dosages is not provided. Was our patient's recurrent ipsilateral AION really the result of arteritic occlusion, or was it a nonarteritic occlusion in a patient with ample arteriosclerotic risk factors? Given that recurrent NAION in the same eye is extraordinarily rare, that no systemic hypotensive episodes were documented, and that the temporal artery biopsy showed dramatically active inflammation, we are persuaded that arteritis accounted for the recurrence. This case illustrates a context in which late biopsy could be useful. There are few other reports regarding late biopsy in the diagnosis and management of GCA. Guevara et al ( 18) reported a patient with recurrence of systemic symptoms 4 months after starting prednisone therapy ( initial dose 60 mg/ day). The prednisone dose at the time of relapse was 30 mg/ d and ESR was within normal limits. No temporal artery biopsy had been obtained at original diagnosis. A biopsy obtained 2 months following the emergence of these new symptoms showed an active, diffuse inflammatory infiltrate and giant cells centered at the internal elas-tica. No further information regarding subsequent follow up is available in this report. Cohen ( 9) described nine patients in whom recurrent systemic symptoms or elevations in ESR occurred each time corticosteroid taper was attempted, despite a year of continuous oral prednisone therapy ( initial dose 40- 100 mg/ day). Because several patients had already experienced corticosteroid complications ( Cushing syndrome, gastrointestinal bleeding, osteoporosis), each received a repeat biopsy at 1 year after diagnosis to better determine which patients had persistent active arteritis. Four patients in this group had a negative biopsy ( vessel fibrosis but no active inflammatory infiltrate or giant cells) and were tapered off prednisone. None had any recurrence of symptoms or elevated ESR during a follow- up period ranging from 5 months to 2 years. The remaining five patients had active histopathology ( diffuse intramural inflammatory infiltrate with giant cells and destruction of the internal elastica). Of these, one patient discontinued corticosteroid therapy at the time of biopsy and had a prompt escalation of systemic symptoms and increased ESR that resolved when treatment was restarted. The other four biopsy- positive patients were continued on prednisone treatment of another year. Three patients in this group, as well as the patient who had briefly discontinued therapy, were subsequently tapered from prednisone without recurrence of GCA after a follow- up of at least 5 months. The remaining patient continued to demonstrate an intermittently elevated ESR with each attempt at reducing the prednisone. One year after the second biopsy, he underwent a third temporal artery biopsy. It was negative, so he was tapered off prednisone without a relapse after 2 years of follow- up. These studies ( 9,18) suggest that late biopsy may be useful in indicating whether prednisone therapy should be continued despite its many serious side effects. Although we acknowledge that late arteritic AION is rare, our case emphasizes that it may occur even when corticosteroid treatment has eliminated systemic symptoms and normalized acute phase reactants. These indicators are evidently not an adequate guide to long- term treatment. The fact that visual loss and a floridly positive biopsy were documented 13 months after diagnosis in our case supports an accumulating anecdotal literature suggesting that patients may need to be protected with higher prednisone doses for well over 1 year in GCA. In patients who are vulnerable to corticosteroid side effects, where corticosteroids must be tapered relatively quickly, late biopsy may be a reasonable option in guiding the tapering process. REFERENCES 1. Klein RG, Hunder GG, Stanson AW, et al. Large artery involvement in giant cell ( temporal) arteritis. Ann Intern Med 1975; 83: 806- 12. 2. Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990; 33: 1122- 8. 3. Horton BT, Magrath TB, Brown GE. An undescribed form of arteritis of the temporal vessels. Mayo Clin Proc 1932; 7: 700. 4. Palm E. The ocular crisis of the temporal arteritis syndrome ( Horton). Acta Ophthalmol 1958; 36: 208^ 13. 5. Henkind P, Charles NC, Pearson J. Histopathology of ischemic optic neuropathy. Am J Ophthalmol 1970; 69: 78- 90. 6. Keltner JL. Giant- cell arteritis. Signs and symptoms. Ophthalmology. 1982; 89: 1101- 10. 7. Gonzalez- Gay MA, Garcia- Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine 2000; 79: 283- 92. 8. Cullen JF. Occult temporal arteritis. A common cause of blindness in old age. Br J Ophthalmol 1967; 51: 513- 25. 9. Cohen DN. Temporal arteritis: improvement in visual prognosis and mnagement with repeat biopsies. Trans Am Acad Ophthalmol Otolaryngol 1973; 77: 74- 85. 10. Beri M, Klugman MR, Kohler JA, et al. Anterior ischemic optic neuropathy VII. Incidence of bilaterality and various influencing factors. Ophthalmology 1987; 94: 1020- 8. 11. Liu GT, Glaser JS, Schatz NJ, et al. Visual morbidity in giant cell arteritis. Clinical characteristics and prognosis for vision. Ophthalmology 199' 4; 101: 17'' 9- 85. 12. CalamiaKT, Hunder GG. Clinical manifestations of giant cell ( temporal) arteritis. Clin Rheum Dis 1980; 6: 389- 403. 13. Aiello PD, Trautmann JC, McPhee TJ, et al. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100: 550- 5. 14. Fauchald P, Rygvold O, Oystese B. Temporal arteritis and polymyalgia rheumatica: clinical and biopsy findings. Ann Intern Med 1972; 77: 845- 52. 15. Fulton AB, Lee RV, Jampol LM, et al. Active giant cell arteritis with cerebral involvement. Findings following four years of corticosteroid therapy. Arch Ophthalmol 1976; 94: 2068- 71. 16. Murgatroyd H, Milne A. Positive temporal artery biopsy in a patient on therapeutic doses of steroids for six years. Eye 2001; 15: 250- 1. 17. Krall PL, Mazanec DJ, Wilke WS. Methotrexate for corticosteroid-resistant polymyalgia rheumatica and giant cell arteritis. Cleve Clin J Med 1989; 56: 253- 7. 18. Guevara RA, Newman NJ, Grossniklaus HE. Positive temporal artery biopsy 6 months after prednisone treatment. Arch Ophthalmol 1998; 116: 1252- 3. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 125 JNeuro- Ophthalmol, Vol. 23, No. 2, 2003 Kim et al. 19. Newman P. Giant cell arteritis with normal sedimentation rate. Arch Neurol 1978; 35: 620. 20. Park JR, Jones JG, Hazleman BL. Relationship of the erythrocyte sedimentation rate to acute phase proteins in polymyalgia rheumatica and giant cell arteritis. Ann Rheum Dis 1981 ; 40: 493- 5. 21. Biller J, Asconape J, Weinblatt ME, et al. Temporal arteritis associated with normal sedimentation rate. JAMA 1982; 247: 486- 7. 22. Ellis ME, Ralston S. The ESR in the diagnosis and management of polymyalgia rheumatica/ giant cell arteritis syndrome. Ann Rheum Dis 1983; 42: 168- 70. 23. Kyle V, Cawston TE, Hazleman BL. Erythrocyte sedimentation rate and C- reactive protein in the assessment of polymyalgia rheumatica/ giant cell arteritis on presentation and during follow up. Ann Rheum Dis 1989; 48: 667- 71. 24. Andersson R, Malmvall BE, Bengsston BA. Acute- phase reactants in the initial phase of giant cell arteritis. Acta MedScand 1986; 220: 365- 7. 25. Hachulla E, Saile R, Parra HJ, et al. Serum amyloid- A concentrations in Giant- cell arteritis and polymyalgia rheumatica: a useful test in the management of the disease. Clin Exp Rheumatol 1991; 9: 157- 63. 26. Gudmundsson M, Nordborg E, Bengtsson B A, et al. Plasma viscosity in giant cell arteritis as apredictor of disease activity. Ann Rheum Dis 1993; 52: 104- 9. 27. Faarvang KL, Pontoppidan Thyssen E. Giant cell arteritis: loss of vision during corticosteroid therapy. JIntMed 1989; 225: 215- 6. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 126 © 2003 Lippincott Williams & Wilkins |