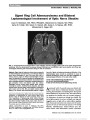
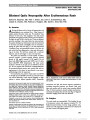
| OCR Text |
Show Correlation of Clinical Profile and Specific Histopathological Features of Temporal Artery Biopsies Rebecca C. Stacy, MD, PhD, Aubrey L. Gilbert, MD, PhD, Joseph F. Rizzo III, MD Background: This study sought to correlate the clinical features of patients with giant cell arteritis (GCA) who present with ophthalmic symptoms and signs, with 2 specific histopathological findings-the presence of giant cells and arterial wall neoangiogenesis. The goal was to assess if these pathological features might be useful in guiding the approach to patient management. Methods: Medical charts were retrospectively reviewed from 58 patients who underwent a temporal artery biopsy at a single institution. Detailed information was collected about the clinical presentation and course, with an empha-sis on visual function. Histopathological and immunohisto-chemical techniques were used to examine temporal artery biopsies for evidence of inflammation. Correlations were made between the clinical data and the presence of giant cells and neoangiogenesis. Results: Twenty-one (34%) biopsies were positive for inflammation consistent with GCA. Although the percentage of positive biopsies with giant cells was high, neither the presence of giant cells nor neoangiogenesis was predictive of a patient's presenting visual symptoms, severity and bi-laterality of vision loss, other ophthalmic manifestations of GCA, presence of headache or jaw claudication, or erythro-cyte sedimentation rate. Giant cells were more common in patients with recent weight loss. Immunohistochemistry confirmed diagnoses but did not alter the clinical course or treatment plan. Conclusions: There was no correlation between the clinical, specifically visual, features of GCA and the presence or absence of giant cells or neoangiogenesis in temporal artery biopsy specimens. Although the presence of neo-angiogenesis may be important in the pathogenesis of GCA, our study showed no correlation between this finding and the clinical course. Journal of Neuro-Ophthalmology 2015;35:127-133 doi: 10.1097/WNO.0000000000000213 © 2015 by North American Neuro-Ophthalmology Society Giant cell arteritis (GCA) is a systemic vasculitis that can cause permanent visual loss in older adults, although the majority of patients with GCA do not have visual symp-toms (1-5). Despite some controversy, biopsy of the tem-poral artery is the gold standard to establish a diagnosis of GCA (6,7). The typical defining characteristics of posi-tive biopsies include T lymphocytes, epithelioid histiocytes, and possibly multinucleated giant cells, although the latter are not required for the diagnosis (6,8). A positive biopsy may additionally demonstrate intimal hyperplasia, luminal narrowing, neoangiogenesis, and internal elastic lamina fragmentation (9,10). More recently, the use of immuno-histochemical stains to identify inflammation has been sug-gested as a strategy to help to resolve diagnostic uncertainty (11,12). However, the question remains whether specific histopathological features, such as giant cells or neoangio-genesis, have clinical significance or implications for patient management. The goal of our study was to provide assessment of any correlations between the visual outcomes of patients with GCA and histopathological findings of temporal artery biopsy. Such data are important as new steroid-sparing therapies are being explored that target specific immune mechanisms (13). METHODS Permission for review and analysis of medical records and biopsy specimens for this study was granted by the Institutional Review Board at Massachusetts Eye and Ear Infirmary (MEEI), Boston, MA. A retrospective blinded chart review was undertaken without knowledge of the biopsy results for all patients with complete medical records Department of Ophthalmology (RCS, ALG, JFR), Harvard Medical School, Boston, Massachusetts; Division of Neuro-Ophthalmology (RCS, JFR), Massachusetts Eye and Ear Infirmary, Boston, Massa-chusetts; and Division of Ocular Pathology (RCS), Massachusetts Eye and Ear Infirmary, Boston, Massachusetts. The authors report no conflicts of interest. R. C. Stacy and A. L. Gilbert contributed equally to the article. Address correspondence to Rebecca C. Stacy, MD, PhD, 243 Charles Street, David G. Cogan Laboratory of Ocular Pathology, 3rd Floor, MEEI, Boston, MA 02114; E-mail: rebecca_stacy@meei. harvard.edu Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 127 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. from MEEI who underwent temporal artery biopsy between January 2008 and April 2013. All patients had been examined either in the neuro-ophthalmology or oculoplastic clinic and had temporal artery biopsies performed by MEEI oculoplastic surgeons. All initial histopathological diagnoses had been rendered by 1 of 3 MEEI ocular pathologists. Data about ophthalmic presentation and findings included initial visual acuity, visual acuity at last follow-up visit, color vision, funduscopic examination, visual field test result, presence of an ischemic disease involving the eye or visual pathways, or ocular motor cranial nerve palsies. Presenting symptoms were categorized according to the presence or absence of permanent visual loss, transient visual loss, or diplopia. Ophthalmic signs were detailed about visual acuity worse than 20/400, bilaterality of vision loss, optic neuropathy, and central retinal artery occlusion (CRAO). The range of normal values for C-reactive protein varied significantly because many of the initial tests were performed in different laboratories. The specimen slides from all 58 biopsies were re-evaluated in a blinded fashion by 2 of the authors (R.C.S., A.L.G.) with no knowledge of the previous diagnoses or clinical presentation. All specimens were evaluated with hematoxylin and eosin and an elastin stain. In some cases, immunohis-tochemistry had already been obtained at the time of diagnosis. All other specimens were stained with the same battery of immunohistochemical stains used for the other specimens, which included CD3 (DAKO, Carpinteria, CA) for T cells and CD68 (Leica, Wetzlar, Germany) or CD163 (Leica) for histiocytes. Neoangiogenesis was highlighted by staining for CD31 (DAKO), which is an endothelial cell marker. A positive diagnosis was made according to accepted criteria (9,12), based on the presence of intimal hyperplasia and transmural inflammation and confirmed using immunohistochemistry-assisted modified criteria (12). To determine statistical significance, student 2-tailed t tests were performed for continuous variables, and Fisher exact tests were used for dichotomous variables. RESULTS Clinical Data Of the 58 biopsies available for review, 21 were positive and 37 were negative; the diagnoses made upon our rereview matched the diagnoses that had been assigned at the time of the initial evaluation. The overall diagnosis of positive or negative GCA also did not change with the information provided by immunohistochemical staining. All histopath-ological diagnoses concurred with clinical diagnoses that were made by the treating physician. Clinical data and statistical analyses for patients with positive and negative biopsies are summarized in Table 1. A significant difference was found in the degree of vision loss between categories: 11 patients (82%) with positive bio-psies had acuities in the affected eye of 20/400 or worse, whereas 9 patients (35%) with negative biopsies had acuities of 20/400 or worse. Three patients with positive biopsies had bilateral vision loss, whereas none of the patients with negative biopsies had bilateral vision loss. There also was a statistically significant difference in the prevalence of jaw claudication, erythrocyte sedimentation rate, and platelet count. In the 21 patients with positive biopsies, the most common cause of visual loss was ischemic optic neurop-athy, which was found in 10 patients (48%), followed by CRAO, found in 3 patients (14%) (Table 1). Four patients with negative temporal artery biopsies were diag-nosed with "biopsy-negative" GCA based on clinical sus-picion. Of the remaining 33 patients with negative temporal artery biopsies, final diagnoses were available for 18 patients. The most common alternative diagnosis to explain vision loss was a CRAO due to atheroembolic disease, which was diagnosed in 9 patients (17%) (i.e., 50% of patients with known alternative diagnoses) and nonarteritic ischemic optic neuropathy was diagnosed in 3 patients. All 21 patients (100%) with positive biopsies were treated with corticosteroids, either methylprednisolone or prednisone. Twenty-eight patients (76%) with negative biopsies had been treated with prednisone before or on the day of their biopsy. The time lag between treatment and biopsy was not significantly different between the 2 groups. Only 1 patient in the positive biopsy group had any improvement in vision. This patient had a CRAO and had been started on 60 mg of prednisone 2 weeks after her acuity was found to be 20/400. After 2 weeks of prednisone, her vision improved to 20/90 and remained stable for 4 months during the period of prednisone taper. Histopathological Findings in Positive Biopsies and Clinical Correlations Positive biopsies were divided into categories based on the presence or absence of giant cells and neoangiogenesis. The mean length of the postfixation biopsy specimen was 1.4 cm. There also was no statistical difference in length of positive biopsy specimens with or without giant cells or neoangiogenesis. There also was no significant difference in time lag from the introduction of corticosteroid treatment to biopsy between any compared categories. Inflammatory changes included giant cells detected with routine staining techniques (Fig. 1A) and with immunohis-tochemistry (Fig. 1B). Use of immunohistochemical stains confirmed and localized inflammation for 4 positive biop-sies in which inflammation was more subtle (Fig. 1C and 1D). Immunohistochemical stain CD31 highlighted neo-angiogenesis that may not have been apparent with hema-toxylin and eosin (Fig. 1E). 128 Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. None of the negative biopsies had notable inflammation within the vessel wall, including any giant cells or signs of neoangiogenesis even after immunohistochemical staining. Within the group of patients with positive biopsies, there were 17 specimens (80%) that contained giant cells and 4 specimens (20%) that did not. There were no significant clinical differences between the 2 groups regarding the following variables: age, gender, presenting ophthalmic symptom, presence of concurrent headache, jaw claudica-tion, history of polymyalgia rheumatica, history of stroke, erythrocyte sedimentation rate, time from steroid treatment to biopsy, percentage of patients with vision loss to an acuity of 20/400 or worse, bilateral vision loss, or percentage of patients with the diagnosis of ischemic optic neuropathy or CRAO (Table 2). Platelet values were not available for 3 of the 4 patients with negative biopsies, so this parameter was not statistically compared. The only statistically significant finding was the presence of weight loss, which was found in 11 patients who had positive biopsies containing giant cells but in no patient with giant cell-negative inflammation. Similar results were seen when comparing groups with and without neoangiogenesis. Specifically, 15 specimens (71%) had neoangiogenesis within the vessel wall, and 6 specimens (29%) did not. As above, there were no significant clinical differences (Table 2), and weight loss was not found to be significantly differ-ent in this comparison. When comparing other histological parameters from the positive specimens, there was a statistically significant association between giant cells and neoangiogenesis (P = 0.05) (Table 2). The converse was also true. All but 3 speci-mens had visible inflammation in all 3 layers of the vessel wall; statistically, transmural inflammation was not more common in specimens with giant cells or neoangiogenesis. However, there was a trend without significance of trans-mural inflammation with neoangiogenesis (P = 0.07). No statistical correlation was seen for the presence or absence of accessory vessel inflammation regarding giant cells or neo-angiogenesis. The internal elastic lamina was fragmented in all positive biopsies. DISCUSSION Our study is one of a small number attempting to correlate clinical and histopathological findings in temporal artery biopsy specimens from an ophthalmic and neuro-ophthalmic perspective (12,14,15). Our patient population and focus of clinical examinations differs significantly from TABLE 1. Clinical data of patients with positive and negative temporal artery biopsies Positive Biopsy Negative Biopsy P Value Number of patients 21 37 Age, mean, yr 76 73 0.17 Female/male 14/7 (67%/33%) 18/19 (49%/51%) 0.27 Presenting visual symptom Permanent vision loss 13 (61%) 26 (70%) 0.57 Bilateral vision loss 3 (14%) 0 0.04 Transient vision loss 3 (14%) 6 (16%) 1.00 Diplopia 3 (14%) 3 (8%) 0.66 Constitutional symptoms Headache 9 (43%) 17 (46%) 1.00 Jaw claudication 9 (43%) 4 (11%) 0.01 Weight loss 11 (52%) 10 (27%) 0.09 History of polymyalgia rheumatica 3 (14%) 2 (5%) 0.34 History of stroke 4 (19%) 3 (8%) 0.24 Examination finding Visual acuity #20/400 11 (82%)* 9 (35%)* 0.04 Optic neuropathy 10 (48%) 3 (17%)† 0.02 CRAO 3 (14%) 9 (50%)† 0.07 Laboratory studies Erythrocyte sedimentation rate (mean) 77 44 0.02 Platelet count, ·1000 (mean) 386 287 0.002 Number of days from initiation of steroid treatment to temporal artery biopsy (mean, median) 4, 2 9, 3 0.11 Length of biopsy specimen, postfixation, cm (mean) 1.4 1.5 0.54 Bold values are statistically significant. *Percentages based on the number of patients with permanent vision loss. †Percentages based on the 18 patients who had known diagnoses other than biopsy-negative GCA. CRAO, central retinal artery occlusion. Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 129 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 1. Histopathological and immunohistochemical staining of positive temporal artery specimens. A. Change in the vessel wall includes lymphocytic and granulomatous inflammation with giant cells (arrowheads) at the intima-media junction. New vessel formation is present (arrows), and there is severe luminal narrowing (asterisk) (hematoxylin and eosin, · 200). B. T lymphocytes are present throughout the vessel wall (CD3 immunostain, · 200). C. In this specimen, inflammation is obvious in the adventitia (arrow) but not elsewhere (hematoxylin and eosin, · 100). D. Immunostaining of the vessel shown in (C) reveals inflammation along the internal elastic lamina (inset, arrow) (CD163 immunostain, · 100). E. In another specimen, immunohistochemical staining confirms the presence of neoangiogenesis in the vessel wall (CD31 stain, · 100). 130 Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. most other research in this area, which typically mines data from general medical or rheumatological clinics, where only 30%-50% of patients report visual complications (1,2,4,16,17). In our survey, 54 of 58 patients were referred for visual symptoms, and 67% of our patients had permanent visual loss. However, the patients we studied otherwise matched the profile generally reported in the medical literature for GCA, having similar demographics of age and gender, similar clinical manifestations, and visual symptoms and signs (1,3-5,15,17-19). The absence of visual recovery seen among all but 1 of our patients is consistent with the poor visual prognosis in GCA (1,3,20). Although the prevalence of headache was less than reported from other studies (5,17,18,21,22), our patient cohort is quite similar to those described in the literature. This study sought to correlate the clinical features of patients having GCA with 2 specific histopathological findings, the presence of giant cells and arterial wall neoangiogenesis, to assess if these pathological features might be useful in guiding the approach to management. Giant cells and CD68+ macrophages at the media-intima junction may be the major source of vascular endothelial growth factor (VEGF), which has been implicated in caus-ing neoangiogenesis of the temporal artery (10,23). It has also been suggested that neoangiogenesis may be a protective measure, perhaps lowering the risk of ischemic complica-tions (23,24). However, one study suggests the contrary, that neoangiogenesis is associated with risk of permanent vision loss (2). We chose to focus on the specific histopath-ological parameters of giant cells and neoangiogenesis because of their potential roles in inflammation and/or pro-tection and also to help resolve the different viewpoints on whether they portend a better or worse prognosis. Our results show that in patients referred to an ophthalmologist, the presence or absence of giant cells or neoangiogenesis are not indicators of clinical presentation or course. Our study also demonstrates for the first time that the presence TABLE 2. Clinical and histopathological parameters associated with giant cells and neoangiogenesis within the temporal artery specimens With Giant Cells Without Giant Cells P Value With NA* Without NA* P Value Number of patients 17 4 15 6 Age, mean, yrs 76 75 0.74 76 75 0.82 Female 12 (71%) 3 (75%) 1.00 10 (67%) 4 (67%) 1.00 Presenting symptom Permanent vision loss 12 (71%) 1 (25%) 0.25 10 (67%) 3 (50%) 0.63 Bilateral vision loss 3 (18%) 0 1.00 2 (13%) 1 (17%) 1.00 Transient vision loss 1 (6%) 2 (50%) 0.48 2 (13%) 1 (17%) 1.00 Diplopia 1 (6%) 2 (50%) 0.08 2 (13%) 1 (17%) 1.00 Concurrent symptoms Headache 8 (47%) 1 (25%) 0.60 7 (47%) 2 (33%) 0.66 Jaw claudication 7 (41%) 2 (50%) 1.00 8 (53%) 1 (17%) 0.18 Weight loss 11 (65%) 0 0.04 9 (60%) 2 (33%) 0.36 History of polymyalgia rheumatica 2 (13%) 1 (25%) 0.48 2 (13%) 1 (17%) 1.00 History of stroke 3 (18%) 1 (25%) 1.0 3 (20%) 1 (17%) 1.00 Examination finding Visual acuity #20/400† 10 (83%)† 1 (100%)† 1.00 8 (53%)† 3 (50%)† 1.00 Optic neuropathy 7 (41%) 1 (25%) 1.00 7 (47%) 3 (50%) 1.00 CRAO 3 (18%) 0 0 3 (20%) 0 0.52 Laboratory studies Erythrocyte sedimentation rate (mean) 77 60 0.43 81 64 0.11 Platelet count, · 1000 (mean) ‡ ‡ ‡ 403 333 0.09 Days from initiation of steroid treatment to temporal artery biopsy (mean, median) 4, 2 4, 2 0.98 4, 2 2, 1 0.31 Transmural inflammation 17 (100%) 3 (75%) 0.19 15 (100%) 4 (50%) 0.07 Accessory vessel involvement 8 (47%) 1 (25%) 0.60 5 (33%) 4 (67%) 0.33 Giant cells - - - 14 (93%) 3 (50%) 0.05 NA 14 (82%) 1 (25%) 0.052 - - - Bold value is statistically significant. *NA, neoangiogenesis. †Percentages based on the number of patients with permanent vision loss. ‡Not analyzed due to insufficient data (see text). CRAO, central retinal artery occlusion. Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 131 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. of angiogenesis is not a guaranteed reprieve from devastating visual complications. Some additional positive findings among the data were discovered: giant cells and neoangiogenesis were correlated (albeit not to a significant extent). This may be related to the increased levels of vasogenic substances, such as VEGF and interferon-g that are found in arteries infiltrated with macrophages (10). We also found one constitutional symp-tom that was associated with histopathology: weight loss was correlated with giant cells. Interestingly, weight loss was found to be negatively associated with irreversible vision loss in another study (1), so the significance of this finding remains to be elucidated. Our overall findings are consistent with but expand upon several other studies. Bevan et al (16) found that giant cells were not correlated with vision loss. Similar studies also showed that giant cells were not indicative of visual symptoms or optic nerve involvement, but these reports did not provide measurements of visual function (15,24,25). Yet another study suggested that the depth of inflammation in the tem-poral artery was possibly related to visual symptoms, although the presence of giant cells was not (14). Another report came to an opposite conclusion that giant cells were related to permanent vision loss (2). However, in this series of more than 300 patients, fewer than 10% of patients had vision loss and 40% of patients had negative biopsies. There are several potential limitations to our study. although our specimens were of standard length for histo-pathological analysis, focal areas of inflammation can be as small as 330 mm (26), and variability within an artery is common (26). Because of the possibility of "skip lesions," it is thought that a longer specimen increases the biopsy yield (27). However, various surgical subspecialists may have dif-ferent surgical approaches to temporal artery biopsies. For example, in one survey from 3 institutions, ophthalmologists who performed temporal artery biopsies sampled longer speci-mens than other types of surgeons (28). In our study, all biopsies were obtained by ophthalmic plastic surgeons trained in ophthalmology. The mean specimen length in our survey, 1.4-cm postfixation, exceeded the critical value found to be associated with a meaningful result (28). Notably, specimen lengths were not provided in other studies that correlated clinical presentation with histopathological findings. Interobserver and intraobserver variability in temporal artery biopsy diagnosis has been reported to be as high as 25% (9) and could be another limitation to our study. In our series, all pathological diagnoses were confirmed by a second blinded review and the interpretations matched the original diagnoses of whether there was evidence or not of active inflammation in the temporal artery. This high correlation might be due, in part, to the use of immuno-histochemistry supporting the findings of Zhou et al (12). In our series, immunohistochemistry confirmed and did not change the diagnosis when the original biopsy was reviewed without special stains. One potential confounding factor that might have influenced our results involves treatment: patients with vision loss were often treated with corticosteroids at the first hint of the diagnosis and biopsied on average 2 days later. This short duration of treatment is unlikely to alter the histopathological diagnosis because the inflammatory response is usually robust within the first 2 weeks (20,29). There were no significant differences in treatment periods for all groups compared in this study. In summary, patients with GCA who present with visual symptoms show no correlation between the clinical features of their presentation and the presence or absence of giant cells or neoangiogenesis of the temporal artery biopsy specimen. Our study is the first to show that neoangio-genesis, which has been suggested to be a protective measure, does not correlate with the severity of the visual or general clinical presentation. As often discussed (13), identification of biomarkers (either in the temporal artery biopsy or blood) would potentially provide an advantage in tailoring therapy, but this goal remains elusive. REFERENCES 1. Cid MC, Font C, Oristrell J, de la Sierra A, Coll-Vinent B, Lopez- Soto A, Vilaseca J, Urbano-Marquez A, Grau JM. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis, Arthritis Rheum. 1998;41:26-32. 2. Chatelain D, Duhaut P, Schmidt J, Loire R, Bosshard S, Guernou M, Pellet H, Piette JC, Sevestre H, Ducroix JP. Pathological features of temporal arteries in patients with giant cell arteritis presenting with permanent visual loss, Ann Rheum Dis. 2009;68:84-88. 3. Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology. 1993;100:550-555. 4. Gonzalez-Gay MA, Blanco R, Rodriguez-Valverde V, Martinez- Taboada VM, Delgado-Rodriguez M, Figueroa M, Uriarte E. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: predictors and response to treatment, Arthritis Rheum. 1998;41:1497-1504. 5. Seo P, Stone JH. Large-vessel vasculitis. Arthritis Rheum. 2004;51:128-139. 6. Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. 7. Allsop CJ, Gallagher PJ. Temporal artery biopsy in giant-cell arteritis. A reappraisal. Am J Surg Pathol. 1981;5:317-323. STATEMENT OF AUTHORSHIP Category 1: a. Conception and design: R. C. Stacy and J. F. Rizzo; b. Acquisition of data: R. C. Stacy And A. L. Gilbert; c. Analysis and interpretation of data: R. C. Stacy, A. L. Gilbert and J. F. Rizzo. Category 2: a. Drafting the manuscript: R. C. Stacy, A. L. Gilbert and J. F. Rizzo; b. Revising it for intellectual content: R. C. Stacy, A. L. Gilbert and J. F. Rizzo. Category 3: a. Final approval of the completed manuscript: R. C. Stacy, A. L. Gilbert and J. F. Rizzo. 132 Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 8. Morgan GJ Jr, Harris ED Jr. Non-giant cell temporal arteritis. Three cases and a review of the literature. Arthritis Rheum. 1978;21:362-366. 9. McDonnell PJ, Moore GW, Miller NR, Hutchins GM, Green WR. Temporal arteritis. A clinicopathologic study. Ophthalmology. 1986;93:518-530. 10. Kaiser M, Younge B, Bjornsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765-774. 11. Foss F, Brown L. An elastic Van Gieson stain is unnecessary for the histological diagnosis of giant cell temporal arteritis. J Clin Pathol. 2010;63:1077-1079. 12. Zhou L, Luneau K, Weyand CM, Biousse V, Newman NJ, Grossniklaus HE. Clinicopathologic correlations in giant cell arteritis: a retrospective study of 107 cases, Ophthalmology. 2009;116:1574-1580. 13. Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis, Nat Rev Rheumatol. 2013;9:731-740. 14. Diaz VA, DeBroff BM, Sinard J. Comparison of histopathologic features, clinical symptoms, and erythrocyte sedimentation rates in biopsy-positive temporal arteritis, Ophthalmology. 2005;112:1293-1298. 15. Schmidt D, Loffler KU. Temporal arteritis. Comparison of histological and clinical findings, Acta Ophthalmol (Copenh). 1994;72:319-325. 16. Bevan AT, Dunnill MS, Harrison MJ. Clinical and biopsy findings in temporal arteritis, Ann Rheum Dis. 1968;27:271-277. 17. Chmelewski WL, McKnight KM, Agudelo CA, Wise CM. Presenting features and outcomes in patients undergoing temporal artery biopsy. A review of 98 patients. Arch Intern Med. 1992;152:1690-1695. 18. Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. New Engl J Med. 2002;347:261-271. 19. Machado EB, Michet CJ, Ballard DJ, Hunder GG, Beard CM, Chu CP, O'Fallon WM. Trends in incidence and clinical presentation of temporal arteritis in Olmsted County, Minnesota, 1950-1985. Arthritis Rheum. 1988;31:745-749. 20. Cornblath WT, Eggenberger ER. Progressive visual loss from giant cell arteritis despite high-dose intravenous methylprednisolone. Ophthalmology 1997;104:854-858. 21. Hall S, Persellin S, Lie JT, O'Brien PC, Kurland LT, Hunder GG. The therapeutic impact of temporal artery biopsy. Lancet. 1983;2:1217-1220. 22. Cid MC, Hernandez-Rodriguez J, Esteban MJ, Cebrian M, Gho YS, Font C, Urbano-Marquez A, Grau JM, Kleinman HK. Tissue and serum angiogenic activity is associated with low prevalence of ischemic complications in patients with giant-cell arteritis. Circulation 2002;106:1664-1671. 23. Weyand CM, Liao YJ, Goronzy JJ. The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. J Neuroophthalmol 2012;32:259-265. 24. Lie JT. Illustrated histopathologic classification criteria for selected vasculitis syndromes. American College of Rheumatology Subcommittee on Classification of Vasculitis. Arthritis Rheum. 1990;33:1074-1087. 25. Lie JT. The classification and diagnosis of vasculitis in large and medium-sized blood vessels. Pathol Annu. 1987;22(pt 1):125-162. 26. Klein RG, Campbell RJ, Hunder GG, Carney JA. Skip lesions in temporal arteritis. Mayo Clin Proc. 1976;51: 504-510. 27. Gonzalez-Gay MA. The diagnosis and management of patients with giant cell arteritis. J Rheumatol. 2005;32:1186- 1188. 28. Taylor-Gjevre R, Vo M, Shukla D, Resch L. Temporal artery biopsy for giant cell arteritis. J Rheumatol. 2005;32:1279- 1282. 29. Narvaez J, Bernad B, Roig-Vilaseca D, Garcia-Gomez C, Gomez-Vaquero C, Juanola X, Rodriguez-Moreno J, Nolla JM, Valverde J. Influence of previous corticosteroid therapy on temporal artery biopsy yield in giant cell arteritis. Semin Arthritis Rheum. 2007;37:13-19. Stacy et al: J Neuro-Ophthalmol 2015; 35: 127-133 133 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |