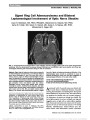
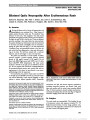
| OCR Text |
Show Retinal Atrophy in Eyes With Resolved Papilledema Detected by Optical Coherence Tomography Brian E. Goldhagen, MD, M. Tariq Bhatti, MD, Pratul P. Srinivasan, Stephanie J. Chiu, PhD, Sina Farsiu, PhD, Mays A. El-Dairi, MD Background: To apply automated spectral domain optical coherence tomography (SD-OCT) segmentation to eyes with resolving papilledema. Methods: Ninety-four patients with idiopathic intracranial hypertension seen at the Duke Eye Center neuro-ophthalmology clinic between November 2010 and October 2011 were reviewed. Excluded were eyes with papilledema with Frisén grade .2, other optic neuropathies or retinopathies, and those that did not have SD-OCT imaging. The remaining 43 patients were split into 2 groups: non-atrophic papilledema and atrophic papilledema. Automated SD-OCT segmenta-tion was performed on patients with non-atrophic papil-ledema and age-matched controls for each of the 9 regions of the Early Treatment Diabetic Retinopathy Study map. Bonferroni correction was used for multiple comparisons. All SD-OCT scans were reviewed for retinal structural abnormalities. Results: Total macular thickness was significantly thinner within the fovea and inner macular ring in non-atrophic papilledema vs control eyes (266 vs 276 mm, P = 0.04; 333 vs 344 mm P , 0.01, n = 26 non-atrophic papil-ledema, 30 controls). SD-OCT demonstrated thinning within the fovea, inner macular ring, and outer macular ring of the outer plexiform layer plus nuclear layer in non-atrophic papilledema vs control (124 vs 131 mm, P , 0.01; 112 vs 118 mm, P = 0.03; 95 vs 100 mm, P = 0.03). Retinal structural changes were seen in 21/33 eyes with atrophic papilledema vs none of the eyes with non-atrophic papilledema or controls. Conclusions: SD-OCT shows qualitative and quantitative changes in the macula of eyes with resolved papilledema. Journal of Neuro-Ophthalmology 2015;35:122-126 doi: 10.1097/WNO.0000000000000210 © 2015 by North American Neuro-Ophthalmology Society Papilledema is defined as optic nerve swelling caused by increased intracranial pressure and can lead to profound irreversible vision loss due to secondary optic atrophy (1,2). One of the common causes of papilledema in young adults is idiopathic intracranial hypertension (IIH). In this study, IIH was defined using the Modified Dandy criteria (3). IIH is an ideal disease model to study optic nerve damage caused by papilledema since confounding causes of optic atrophy, such as compression or inflammation, are not present. When papilledema occurs, there are changes in pressure gradients caused by increased subarachnoid pressure that lead to impairment of axoplasmic flow within the optic nerve resulting in swelling of axons (manifesting as optic disc edema) (4,5). The eventual resultant loss of axonal integrity leads to loss of axons and their retinal ganglion cells (i.e., optic atrophy) (6). There is no study to date that has been able to distinguish resolution of papilledema from superim-posed axonal loss or optic atrophy in treated cases of IIH. Automated spectral domain optical coherence tomogra-phy (SD-OCT) segmentation accurately identifies retinal layer boundaries in normal patients and patients with retinal pathology (7-10). This technique has been used in patients with neuro-ophthalmic disease, and it has been suggested that analysis of the ganglion cell layer plus inner plexiform layer (GCL-IPL) is more predictive of disease progression and visual loss than measurement of the retinal nerve fiber layer (RNFL) (11,12). In this study, we evaluated the thickness of the macula and its layers using automated SD-OCT segmentation in eyes of adult patients with stable, mild chronic papilledema due to IIH. We hypothesized that automated SD-OCT segmentation could be used to detect subclinical retinal or optic atrophy in this patient cohort. Departments of Ophthalmology (BEG, MTB, SF, MAE), Neurology (MTB), and Biomedical Engineering (PPS, SJC, SF), Duke University Medical Center, Durham, North Carolina. Supported by a grant from the Research to Prevent Blindness departmental fund. The authors report no conflicts of interest. Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www. jneuro-ophthalmology.com). Address correspondence to Mays A. El-Dairi, MD, Duke Eye Cen-ter, 2351 Erwin Road, PO box 3802, Durham, NC, 27710; E-mail: mays.el-dairi@duke.edu 122 Goldhagen et al: J Neuro-Ophthalmol 2015; 35: 122-126 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. METHODS This study was designed as a retrospective chart review. It was approved by the Duke University Medical Center Institutional Review Board and conducted in accordance with the standards of the Health Insurance Portability and Accountability Act. The Duke Enterprise Data Unified Content Explorer (D.E.D.U.C.E.) was used to identify patients aged 16 years and older seen at the Duke Eye Center neuro-ophthalmology clinic between November 2010 and October 2011 with the ICD-9 diagnosis of 377.00 (Papilledema NOS) and 348.2 (Benign intracranial hypertension). Patients who did not have SD-OCT data collected by Heidelberg Spectralis (required for the automated SD-OCT segmentation protocol as described by Chiu et al (7,9)) were excluded. Also excluded from the study were patients who had other conditions that may affect the optic nerve head (ONH) including glaucoma, ONH drusen, and large refractive error (spherical equivalent of greater than ±5 di-opters or astigmatism of greater than 3 diopters). One patient with fulminant IIH was excluded because she was lost to follow-up 2 weeks after optic nerve sheath fenestration. Data gathered on each patient included age, gender, race, ocular and medical history, visual acuity, visual fields results using the Humphrey Field Analyzer/HFA II-i (Carl Zeiss Meditec, Dublin, CA), appearance of the ONH, color vision, and raw E2E data from Spectralis OCT (Heidelberg, Carlsbad, CA) horizontal linear scans of the macula and circular scan of the peripapillary region. We included eyes with resolved or resolving papilledema looking for atrophic changes; eyes with moderate or severe papilledema (Frisén grade .2) were not included in this study. The eyes in our study were subdivided into 2 groups: atrophic papilledema and non-atrophic papilledema (Frisén grade #2) (Table 1). Atrophic papilledema was defined as visible ONH pallor. All patients with ONH pallor had some form of vision loss (i.e., reproducible visual field changes other than an enlarged blind spot and/or best corrected visual acuity ,20/40). Non-atrophic papilledema was defined as stable disease without evidence of ONH pallor, RNFL thickness $80 mm, and normal visual field testing (mean deviation less than 23.0 dB on Humphrey 24-2 SITA standard visual field testing and no visual field defect other than an enlarged blind spot). SD-OCT scans from both groups were reviewed for any retinal structural abnormalities. These structural abnormal-ities included epiretinal membranes (ERMs), inner or outer retinal layer cysts, and photoreceptor (PR) loss. SD-OCT scans from the non-atrophic papilledema group as well as the control group were segmented by Duke Optical Coherence Tomography Retinal Analysis Program (DOC-TRAP) software using a graph-based automated segmenta-tion analysis protocol which was previously validated for images of various disease pathologies (7-10). The eye with the more reliable OCT or thinner RNFL was selected for comparison in these patients, with the exception of when that eye had met 1 or more of the exclusion criteria. In in-stances where there were multiple visits during the period of November 2010 and October 2011, only scans from the most recent visit were used. Normal controls were age-matched to patients in the mild non-atrophic papilledema group (Table 1). Thickness was determined for RNFL, GCL-IPL, inner nuclear layer (INL), outer plexiform layer plus outer nuclear layer (OPL-ONL), inner plus outer photoreceptor segments (IS-OS), and retinal pigment epithelium (RPE) (Fig. 1). The Early Treatment Diabetic Retinopathy Study (ETDRS) map was used to divide the macula into 9 regions consisting of 3 concentric circles with diameters measuring 1 mm (fovea), 3 mm (inner ring), and 6 mm (outer ring). Peripapillary RNFL thickness was acquired from the Heidelberg Spectralis OCT TABLE 1. Demographic data of patients software. demonstrating atrophic papilledema, nonatrophic papilledema, and controls Atrophic Papilledema Non-Atrophic Papilledema Controls N 17 26 30 Age, yr 37 (±16) 32 (±9) 33 (±15) Gender 16 f/1 m 25 f/1 m 23 f/7 m Race 4 w/5 b 7 w/18 b 14 w/7 b Presenting ONH $3 8 6 Presenting ONH ,3 3 20 Control group was age-matched to non-atrophic papilledema group. Presenting ONH refers to the nerve swelling on the Frisén scale when the patient initially presented for evaluation. SD values within the parenthesis. b, black; f, female; m, male; ONH, optic nerve head; w, white. FIG. 1. Segmentation of spectral domain optical coherence tomography macular scans by Duke Optical Coherence Tomography Retinal Analysis Program (DOCTRAP) demon-strates the vitreous, nerve fiber layer (NFL), ganglion cell layer plus inner plexiform layer (GCL-IPL), inner nuclear layer (INL), outer plexiform layer plus outer nuclear layer (OPL-ONL), inner plus outer photoreceptor segments (IS-OS), retinal pigment epithelium (RPE), and choroid. Goldhagen et al: J Neuro-Ophthalmol 2015; 35: 122-126 123 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Statistical analysis of data was performed using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA). Unpaired Student t tests were used to compare the mild non-atrophic papilledema group with the control group for each of the above-listed automated OCT scan thicknesses. Bonferroni correction was used for multiple comparisons (P-value for each ETDRS segment was multiplied by 8, P-values for each ring was multiplied by a factor of 2). Additionally, the automated DOCTRAP segmentation re-sults were verified by manual evaluation and corrected using DOCTRAP in manual mode if necessary. The error rate comparing the total macular thicknesses within the fovea, inner macular ring, and outer macular ring between the DOCTRAP software and Spectralis software also was performed. RESULTS Ninety-four patients were identified from the D.E.D.U.C.E. query. Forty-five patients were excluded due to not having SD-OCT imaging using the protocol of the study, 4 patients due to ONH swelling .2 on the Frisén scale, and 2 patients due to the presence of ONH drusen. Of the remaining 43 patients, 26 had non-atrophic papilledema (Frisén grade #2) and 17 had atrophic papilledema. Thirty controls were age-matched to those in the non-atrophic papilledema group. Of the 26 patients in the non-atrophic papilledema group, 6 had nerve swelling on initial presentation $3 on the Frisén scale, whereas 20 had nerve swelling ,3. Of the 17 patients in the atrophic papilledema group, 11 initially presented without pallor, of which, 8 had nerve swelling on presentation $3, whereas 3 had nerve swelling ,3 on the Frisén scale (Table 1). There was equivalence of the total macular thicknesses within the fovea, inner macular ring, and outer macular ring between the DOCTRAP software and Spectralis software (average difference 1.13%; range, 0.35%-3.4%). Of the 61 horizontal line scans per patient used to construct the ETDRS map, 35.7% of the scans required slight manual adjustment of at least one of the segmented layers. This adjustment was performed once in a masked manner and only if the DOCTRAP segmentation clearly did not follow the expected contour of a retinal layer. This did not create significant differences in the numerical results for any layer within any ETDRS segment when using the automated vs manually adjusted technique. The total macular thickness was significantly thinner in all papilledema eyes as compared with control within the fovea, inner macular ring, and outer macular ring (263 vs 276 mm, P , 0.01; 323 vs 344 mm, P , 0.001; 288 vs 301 mm, P , 0.01, respectively). Non-atrophic papilledema eyes also showed thinner total macular thickness compared with con-trols within the fovea and inner macular ring (266 vs 276 mm, P = 0.04; 333 vs 344 mm, P , 0.01) (Table 2). Fully automated segmentation demonstrated no difference in layer thickness between all papilledema and control eyes within the RNFL, IS-OS, and RPE. Among these layers, the RNFL was significantly thinner in atrophic papilledema than normal controls within the inner and outer macular ring (25 vs 27 mm, P , 0.01; 36 vs 41 mm, P = 0.03), although there was no difference between non-atrophic papilledema and controls. There was thinning of the GCL-IPL, INL, and OPL-ONL layers in patients with papilledema as compared with controls. Among these layers, the GCL-IPL was thinner among all papilledema patients compared with controls, particularly within the inner and outer macular rings (85 vs 97 mm, P , 0.01; 62 vs 67 mm, P , 0.02). In non-atrophic papilledema patients, there was a trend for reduced thickness within the GCL-IPL inner macular ring as compared with control (93 vs 97 mm, P = 0.12). Significant thinning was seen within the inner macular ring of the INL among all patients with papilledema compared with controls (37 vs 39 mm, P = 0.04). In non-atrophic papilledema patients, there was a trend for reduced thickness within the inner macular ring of the INL when compared with normal controls (37 vs 39 mm, P = 0.13). Within the OPL-ONL layer, there was thinning within the fovea, inner macular ring, and outer mac-ular ring among all patients with papilledema vs controls (124 vs 131 mm, P , 0.01; 112 vs 118 mm, P = 0.01; 95 vs 100 mm, P , 0.01). This was also the case in each of these regions among those patients with non-atrophic papilledema when compared with controls (124 vs 131 mm, P , 0.01; 112 vs 118 mm, P = 0.03; 95 vs 100 mm, P = 0.03). Total macular thickness and retinal layers in which there were statistically significant differences for patients with non-atrophic papilledema were further assessed by quadrant. For total macular thickness, the nasal and temporal quadrants of the inner macular ring were thinner as compared with controls (334 vs 348 mm, P , 0.01; 322 vs 335 mm, P , 0.01) (See Supplemental Digital Content, Figure e1, http://links.lww. com/WNO/A124). Within the INL, the nasal quadrant of the inner ring was thinner in mild papilledema as compared with controls (36 vs 38 mm, P = 0.03) (See Supplemental Digital Content, Figure e2, http://links.lww.com/WNO/ A125). Within the OPL-ONL, there was only a trend of thinning in mild papilledema in each quadrant of each mac-ular ring as compared with controls (See Supplemental Dig-ital Content, Figure e3, http://links.lww.com/WNO/A126). There was no difference in thickness between all papil-ledema and controls within the peripapillary RNFL, either centrally or for any quadrant. This also was the case when comparing non-atrophic papilledema vs controls. Thickness of the peripapillary RNFL in eyes with atrophic papilledema was significantly thinner centrally and within the nasal, inferior, and superior quadrants as compared with controls (80 vs 102 mm, P , 0.0001; 56 vs 76 mm, P , 0.01; 108 vs 134 mm, P , 0.05; 88 vs 124 mm, P , 0.0001). Twenty-one of 33 eyes from 17 patients with atrophic papilledema had changes in their retinal architecture. Photoreceptor loss was seen in 17 eyes, INL cysts were 124 Goldhagen et al: J Neuro-Ophthalmol 2015; 35: 122-126 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. seen in 4, ERMs in 9, and a lamellar hole in 1 (Fig. 2). These structural changes persisted with follow-up OCT imaging. Eyes with atrophic papilledema that had retinal structural change had worse visual acuity than eyes with atrophic papilledema with normal retinal architecture (20/ 31 vs 20/23; P = 0.04). All control eyes and those with non-atrophic papilledema had normal retinal architecture. DISCUSSION In this study, we demonstrated that patients with non-atrophic and atrophic papilledema have atrophic changes in the macula. In patients with atrophic papilledema, these changes occur within the inner nuclear, outer nuclear, and photoreceptor layers. Patients with non-atrophic papillede-ma did not demonstrate qualitative structural changes, but we detected quantifiable macular thinning within the inner and outer nuclear layers. Peripapillary RNFL thickness showed no significant difference between eyes with non-atrophic papilledema and normal controls, similar to pre-viously reported studies (13,14). Our findings of thinner nuclear layers in the macula may be a reliable measure to quantify subclinical atrophy in eyes with resolving papille-dema. The overall thinning of the macula in resolving pap-illedema has been reported previously, but segmentation of the individual layers was not performed (15,16). Our secondary findings of outer retinal changes in eyes with atrophic papilledema are particularly intriguing because papilledema is most commonly thought of as a disease of the optic nerve. This would suggest that changes due to papilledema occur within the retina. Potential causes include: 1) mechanical changes at the time of the severe ONH swelling, 2) ischemic changes due to compression of retinal vessels, and 3) retrograde transsynaptic degeneration. Transsynaptic degeneration may occur when injured neurons cause subsequent damage of retinal ganglion cells and inner nuclear neurons, which may be followed by thinning of the OPL and cell loss within the ONL (17). Such changes are detectable on both OCT and electroretinography (18,19) and have been shown to occur after occipital lobe injury, optic neuritis in multiple sclerosis and neuromyelitis optica, and other neurological diseases (17,20-23). Our study is limited by its retrospective and cross-sectional design. We were unable to assess early stages of IIH and control for treatment modality or treatment delay. In addition, we did not assess for mild visual impairment using low-contrast acuity with the Colenbrander Mixed Contrast Eye Chart or with multifocal electroretinography. Our study was also limited by having more than half of potential patients excluded due to not having Spectralis SD-OCT imaging performed as part of their evaluation and management. These TABLE 2. Average thickness (in micrometers) in 6 segmented layers of 3 macular rings in eyes with non-atrophic papilledema vs controls NFL GCL-IPL INL OPL-ONL IS-OS RPE Total Fovea Non-atrophic papilledema 16.3 (2.1) 36.8 (12.7) 20.7 (4.1) 124.3 (9.3) 36.4 (2.6) 28.8 (3.2) 336 (16) Control 16.4 (1.6) 37.0 (9.1) 21.4 (4.2) 131.5 (9.3) 37.7 (3.2) 30.1 (4.0) 346 (11) P value 0.83 0.94 0.53 ,0.01 0.10 0.72 0.04 Inner ring Non-atrophic papilledema 27.2 (1.9) 93.3 (8.0) 37.4 (2.8) 112.0 (9.1) 32.8 (2.6) 29.9 (2.5) 333 (17) Control 27.4 (1.7) 96.6 (4.6) 38.8 (2.7) 117.7 (7.3) 33.4 (3.2) 30.1 (3.4) 344 (10) P value 1.47 0.12 0.13 0.03 0.86 1.53 ,0.01 Outer ring Non-atrophic papilledema 42.4 (5.1) 66.5 (6.4) 30.6 (2.5) 95.4 (8.1) 34.8 (3.4) 25.6 (3.0) 296 (16) Control 41.2 (4.7) 67.2 (4.7) 31.0 (2.5) 100.4 (6.8) 35.1 (2.6) 26.1 (3.1) 301 (12) P value 0.77 1.29 1.20 0.03 1.32 1.17 0.35 SD values within the parenthesis. P-values are Bonferroni corrected. Significant (P , 0.05) are bolded. GCL-IPL, ganglion cell layer plus inner plexiform layer; INL, inner nuclear layer; IS-OS, inner photoreceptor segment plus outer photore-ceptor segment; NFL, nerve fiber layer; OPL-ONL, outer plexiform layer plus outer nuclear layer; RPE, retinal pigment epithelium. FIG. 2. Arrows indicate changes in retinal architecture seen in patients with atrophic papilledema. A. Photoceptor loss. B. Inner nuclear layer cysts. Goldhagen et al: J Neuro-Ophthalmol 2015; 35: 122-126 125 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. limitations could be overcome by performing a continuation study which would involve prospectively enrolling patients, observing them over time from onset of papilledema, and evaluating outcomes with different treatment modalities. Our findings shed light on retinal changes that occur due to papilledema and may help to explain the etiology of vision loss in some patients. Further longitudinal and prospective studies may help to create a model to predict the visual outcome of patients presenting with papilledema. REFERENCES 1. Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS, Hopson D. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39:461-474. 2. Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991;114:155-180. 3. Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5:55-56. 4. Primrose J. Pathogenesis of optic disc swelling. Br J Ophthalmol. 1978;62:579-580. 5. Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol. 1977;95:1553-1565. 6. Grafstein B. The nerve cell body response to axotomy. Exp Neurol. 1975;48:32-51. 7. Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18:19413-19428. 8. Lee JY, Chiu SJ, Srinivasan PP, Izatt JA, Toth CA, Farsiu S, Jaffe GJ. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by cirrus and spectralis systems. Invest Ophthalmol Vis Sci. 2013;54:7595-7602. 9. Chiu SJ, Izatt JA, O'Connell RV, Winter KP, Toth CA, Farsiu S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci. 2012;53:53-61. 10. Maldonado RS, O'Connell R, Ascher SB, Sarin N, Freedman SF, Wallace DK, Chiu SJ, Farsiu S, Cotten M, Toth CA. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012;130:569-578. 11. Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, Crainiceanu CM, Durbin MK, Oakley JD, Meyer SA, Frohman EM, Calabresi PA. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521-533. 12. Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, Conger A, Frohman TC, Newsome S, Ratchford JN, Frohman EM, Calabresi PA. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17:1449-1463. 13. Rebolleda G, Munoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:5197-5200. 14. Kaufhold F, Kadas EM, Schmidt C, Kunte H, Hoffmann J, Zimmermann H, Oberwahrenbrock T, Harms L, Polthier K, Brandt AU, Paul F. Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS One. 2012;7:e36965. 15. Monteiro ML, Afonso CL. Macular thickness measurements with frequency domain- OCT for quantification of axonal loss in chronic papilledema from pseudotumor cerebri syndrome. Eye (Lond). 2014;28:390-398. 16. El-Dairi M, Freedman SF, Asrani S, Buckley E, Bhatti MT. Optical coherence tomography in mildly swollen optic nerves due to idiopathic intracranial hypertension. Paper Presented at: Annual Meeting of the American Academy of Ophthalmology, November 9, 2008; Atlanta, GA. 17. Gills JP, Wadsworth JAC. Retrograde Transsynaptic degeneration of the inner nuclear layer of the retina. Invest Ophthalmol Vis Sci. 1967;6:437-448. 18. Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132:628-634. 19. Monteiro ML, Hokazono K, Cunha LP, Oyamada MK. Correlation between multifocal pattern electroretinography and Fourier-domain OCT in eyes with temporal hemianopia from chiasmal compression. Graefes Arch Clin Exp Ophthalmol. 2013;251:903-915. 20. Jindahra P, Petrie A, Plant GT. The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain. 2012;135:534-541. 21. Gelfand JM, Cree BA, Nolan R, Arnow S, Green AJ. Microcystic inner nuclear layer abnormalities and neuromyelitis optica. JAMA Neurol. 2013;70:629-633. 22. Gills JP Jr. Electroretinographic abnormalities and advanced multiple sclerosis. Invest Ophthalmol. 1966;5:555-559. 23. Jindahra P, Hedges TR, Mendoza-Santiesteban CE, Plant GT. Optical coherence tomography of the retina: applications in neurology. Curr Opin Neurol. 2010;23:16-23. 126 Goldhagen et al: J Neuro-Ophthalmol 2015; 35: 122-126 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |