| OCR Text |
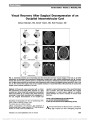
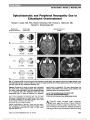
Show Retinal Segmentation Using Multicolor Laser Imaging Robert C. Sergott, MD Abstract: Spectral-domain optical coherence tomography (SD-OCT) changed 3 worlds: clinical care, clinical research, and the regulatory environment of phases 2, 3, and 4 pharmaceutical and surgical trials. OCT is now undergoing another transformation with multicolor technology, which acquires images using data from 3 simultaneous lasers: red, green, and blue, taking advantage of the different wavelengths of each of these colors to most precisely image 3 different zones of the retina. Rather than seeing only the surface of the retina and optic disc and any large lesions in the deeper retina, this technology provides a topographic map of the outer (red), mid (green), and inner (blue) retina somewhat similar to what is observed with fundus autoflourescence of deep retina, retinal pigment epithelium, and choroid. Multicolor imaging will supplement and help to define what is observed with traditional fundus photography and SD-OCT. In addition, it may demonstrate abnormalities when routine photography is normal and when SD-OCT findings are equivocal. This review will illustrate the basic principles of multicolor imaging and will show clinical examples of how this technique can further define retinal and optic nerve pathology. Journal of Neuro-Ophthalmology 2014;34(Suppl):S24-S28 doi: 10.1097/WNO.0000000000000164 © 2014 by North American Neuro-Ophthalmology Society After its description in 1991, optical coherence tomog-raphy (OCT) has fostered a more complete under-standing of the pathological mechanisms of vitreoretinal diseases and has also produced similar insights in neuro-ophthalmology, especially in neurodegenerative disorders. Multicolor laser imaging performed with OCT repre-sents the most recent advance for in vivo retinal imaging. With this technology, the retina and optic nerve are scanned simultaneously with 3 lasers of different wavelengths: infrared (815 nm), green (518 nm), and blue (486 nm). Each colored laser focuses on a different depth within the retina. Because of these different depths of penetration, unique localizing information is obtained from 3 discrete levels of the retina in a topographic map (Fig. 1). The infrared laser penetrates into the deepest retinal layers re-sulting in detailed images of the choroid, retinal pigment epithelium, and photoreceptors. The green laser focuses on the mid-retinal layers and is strongly absorbed by hemo-globin, thereby imaging blood vessels, hemorrhage, and exudates. The blue laser penetrates to the shallowest depth and provides detailed images of the retinal nerve fiber layer, ganglion cells, macular pigment, and any structures on the surface of the retina, such as an epiretinal membrane. Confocal scanning lasers, with real-time eye tracking and "noise" reduction techniques, produce images that are auto-matically color balanced to the appearance of fundus pho-tography. Except for the appearance of the optic disc, the multicolor image is equivalent to the natural color of the retina. Like OCT, multicolor imaging is painless, noncontact, and noninvasive but pupillary dilation is necessary for optimum images. The lasers scan the retina in a continuous manner so that camera alignment can be adjusted through-out the study while confocal optics eliminate scattered light (Fig. 2). METHODS A 5-step approach to the interpretation of multicolor images is recommended (Fig. 3): 1. Examine the color-balanced, composite multicolor image to detect areas of pathologic change. 2. Examine the individual images from each colored laser ("source images"), correlating these findings with the color-balanced image. When evaluating these images, the clinician must always ask whether the images may contain artifacts. Neuro-Ophthalmology Service, Wills Eye Hospital and Thomas Jefferson University, Philadelphia, Pennsylvania. R. C. Sergott is a paid consultant to Heidelberg Engineering as well as the recipient of research funding. He has participated in the development process of several optic nerve and retinal imaging technologies. He does not hold any patents and receives no royalties from Heidelberg Engineering. Address correspondence to Robert C. Sergott, MD, Thomas Jefferson University, Philadelphia, PA 19107; E-mail: rcs220@comcast.net S24 Sergott: J Neuro-Ophthalmol 2014; 34(Suppl): S24-S28 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 3. Assess carefully for evidence of imaging artifacts (see Artifacts section). 4. Correlate the findings of the color-balanced image and the individual laser images and with spectral-domain OCT (SD-OCT) images in the pathological regions. 5. Compare the findings from each eye, correlating with the patient's history and examination findings. ARTIFACTS Artifacts with multicolor laser occur predominantly in the center of the image. Because the lens surfaces are curved, light reflected from the peripheral retina during scanning is scattered out of the beam path and is not captured in the fundus image. The appearance and brightness of the artifact depends on the ratio of the light intensity reflected from the retina, and the intensity of the light reflected on the lens surfaces. No artifact is visible if the pupil is dilated and/or if the media of the patient are clear. However, if a patient has a significant cataract and/or if the camera is improperly aligned an artifact appears. In these cases, the sensitivity of the detector will be increased to obtain a clearly illuminated retinal image, and the light reflected on the lens surface is visible. Patients with corneal opacities, optically significant cataracts, poor pupillary dilation, and high myopia are prone to demonstrate artifacts with multicolor imaging (Fig. 4). Clinical examples are shown below: • Macular Hole (Fig. 5). • Papilledema (Fig. 6). • Nonarteritic anterior ischemic optic neuropathy (Fig. 7). FIG. 1. Traditional fundus photography (left) uses visible, monochromatic light to capture a fundus image. Multicolor laser imaging takes advantage of the differences in penetration of different laser wavelengths from blue, green, and infrared sources to more precisely image and localize pathologic processes in the retina. FIG. 2. Schematic representation of the optics of multicolor laser. Three simultaneous laser colors enter the eye and are reflected back to the beam splitter where the detector pro-cessing the 3 data from the 3 wavelengths and merges them into a composite, color-balanced multicolor image. 1, Telescope; 2, XY scan; 3, Beam splitter; 4, Filter; 5, Detector; 6, Laser. Courtesy of Heidelberg Engineering. Sergott: J Neuro-Ophthalmol 2014; 34(Suppl): S24-S28 S25 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. CONCLUSIONS Multicolor laser imaging represents the next generation of retinal imaging that will further define and localize pathological processes within the retina that are involved in neuro-ophthalmic diseases. Multicolor imaging shares the patient friendly nature of OCT with its noninvasive, noncontact, painless techniques. Proper interpretation of the imaging results depends on correlation with "source images" from the infrared, green, and blue lasers as well as correlation with SD-OCT and the patient's clinical findings. FIG. 3. A, Multicolor composite laser imaging of geo-graphic macular atrophy. B, Infrared image (815 nm) most clearly identifies the border of the geographic lesion with a circumferential white signal, which probably represents the active, advancing edge of the lesion. This imaging in-dicates the geographic atrophy primarily affecting the outer retina, retinal pigment epithelium, and choroid. C, Green laser image (518 nm) shows the lesion somewhat more clearly. The increased reflectivity (white signal) inferiorly and temporally to the fovea indicates involvement of the mid-retinal layers. D, Blue laser (486 nm) image of geo-graphic atrophy shows the overall extent of the lesion but with few details because the pathology is primarily in the deep outer retina. Adapted from Heidelberg Engineering and Professor Sebastian Wolf, Bern, Switzerland. FIG. 4. Central "hot spot" (arrows) is an imaging artifact in a patient with visually significant cataract. The artifact usually is present centrally and in all 3 colored laser images. FIG. 5. Full thickness macular hole imaged with both multicolor technology (A) and spectral-domain optical coherence tomography (SD-OCT) (B). The 2 technologies are complementary. The SD-OCT defines the dimensions of the macular hole and identifies the surrounding cystoid edema with operculum. The multicolor image reveals the full extent of the hole with pathologic changes affecting a large area of the macula. Courtesy of Heidelberg Engineering. S26 Sergott: J Neuro-Ophthalmol 2014; 34(Suppl): S24-S28 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 6. Papilledema. A and B, Spectral-domain optical coherence tomography (SD-OCT) demonstrates optic disc elevation and migration of fluid into the subretinal space (white arrows). Secondary thickening of the inner and mid-retinal layers also is present and disruption of the ellipsoid zone of the photoreceptors in the papillomacular bundle. Multicolor laser image (C) confirms diffuse disc edema and the bright signal in the fovea of the infrared image (D), corresponds to the ellipsoid changes found on SD-OCT. Interestingly, the green and blue laser images (E and F) demonstrate hyperreflectivity (arrows) in a peri-venular location. Sergott: J Neuro-Ophthalmol 2014; 34(Suppl): S24-S28 S27 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 7. Nonarteritic anterior ischemic optic neuropathy (NAION). A, Spectral-domain optical coherence tomography (OCT) demonstrates retinal nerve fiber layer edema in the right eye. The patient has had a prior episode of NAION in the left eye with nerve fiber layer thinning. B, MultiColor image of acute NAION in the right eye (upper left) also demonstrates increased signal in the infrared images of the deep retina inferiorly (upper right) and increased signal in the mid (lower left) and superficial (lower right) retina. C, The left eye with prior NAION has increased signal in the outer retina but dark areas in the superior retina and papillomacular bundle (lower left, lower right) corresponding to the region of retinal nerve fiber layer atrophy seen on the OCT. S28 Sergott: J Neuro-Ophthalmol 2014; 34(Suppl): S24-S28 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |