| OCR Text |
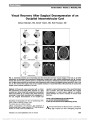
Show Dr. Airy's "Morbid Affection of the Eyesight": Lessons From Teichopsia Circa 1870 Frederick E. Lepore, MD Abstract: Hubert Airy's iconic drawing of his own migraine visual aura for which he coined the term, "teichopsia," con-veys important lessons for the contemporary clinician. His observations of the expansion ("build-up"), minification/ magnification, and color/achromatopsia of migrainous tei-chopsia are consistent with (and possibly anticipatory of) the later discoveries of cortical spreading depression, cor-tical magnification of primary visual cortex (V1), and special-ized cortical centers for color vision. Journal of Neuro-Ophthalmology 2014;34:311-314 doi: 10.1097/WNO.0000000000000133 © 2014 by North American Neuro-Ophthalmology Society When results of detailed physical examination or post-mortem findings were unavailable, the 18th century physician relied exclusively on his powers of observation. Such was the case in 1870, when Hubert Airy (1) created the iconic image of his own visual aura (Fig. 1) recognizable to migraineurs over 140 years later. Although Airy's contribution to the fields of neurology and ophthalmology immeasurably helped to define classic migraine, he never used the term "migraine." As implied by the title, "On a distinct form of Transient Hemiopsia," Airy sought to describe (and illustrate) his episodic "half-blindness." The accuracy of his meticulous observa-tions of movement/expansion, regional miniaturization/ magnification, and color of his visual aura anticipated present-day concepts of visual neuroanatomy and neuro-physiology and provide illuminating lessons for the present-day clinician. Hubert Airy was a 31-year-old Cambridge-matriculated MA, MD, when his account of visual symptoms was read before the Royal Society. Over the preceding 16 years, he had experienced "transient hemiopsia" "much oftener" than a 100 times. His salient observations included: 1. Gradual enlargement over 20 to 25 minutes of a horseshoe-shaped arch with an advancing "zigzag" margin followed by scotoma identically in both eyes. 2. The serrated margin "varied with changing gleams of red and blue and yellow and green, and orange." 3. The size of the "teeth" of the serrated margin were of variable size: "small and fine" near his central point of visual fixation and "larger and larger" with increasing distance from fixation. 4. Near the end of the visual aura, his "headache comes on gradually." (In 1865, his migraineur father, Sir George Airy (2), the Astronomer Royal, described similar obscu-rations of vision without headache). Airy recognized the "inaccuracy and insufficiency" of the term "hemiopsia" and proposed teichopsia (from the Greek "town-wall" and "vision") in its stead. A birds-eye view of the zigzag layout of walls enclosing a medieval fortified town (3) was evocative of "the bastioned form of transient Hemi-opsia" that Airy pictured in his report. LESSON 1: BUILD-UP In Airy's account, his teichopsia was a dynamic process, which would enlarge slowly at first and then more rapidly as it pro-gressed to "gradual occupation of one (lateral) half of the field of view." This build-up of the fortification spectrum is a hall-mark of classic migraine (4) and over 70 years later, led Lashley (5) to speculate on the pathogenesis of the visual aura as he timed the progression of his own "scintillating scotomas." Airy lacked an accurate map of primary visual cortex, and this deficiency was not to be remedied until 13 years after his death by the retinotopic schema of visual cortex derived from Gordon Holmes' (6) study of head wounds during the Great War. Lashley proposed that a "wave of strong excitation" tra-versed 67 mm of visual cortex from occipital pole to rostral calcarine cortex during the 20 minutes' migration of his visual aura, and he calculated the rate of propagation of this distur-bance at 3 mm/min or less. But what was this "wave"? Although the underlying pathophysiology of teichopsia is still unknown, a potential neural mechanism came to light in 1944, when Leao's (7) stimulating bipolar electro-des elicited a wave of depolarization progressing across rab-bit cortex at 3-5 mm/min followed by depression of spontaneous neuronal electrical activity, the phenomenon of cortical spreading depression (CSD). The similar rates of Departments of Neurology and Ophthalmology, Rutgers-Robert Wood Johnson Medical School, New Brunswick, New Jersey. Presented at the Festschrift for Professor Myles Behrens, Edward S. Harkness Eye Institute, Columbia University College of Physicians and Surgeons on May 19, 2012. The authors report no conflicts of interest. Address correspondence to Frederick E. Lepore, MD, Department of Neurology, Rutgers-Robert Wood Johnson Medical School, 125 Pa-terson Street, Room 6210, New Brunswick, NJ 08901; E-mail: leporefe@rutgers.edu Lepore: J Neuro-Ophthalmol 2014; 34: 311-314 311 Historical Note Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. progression of Lashley's aura and Leao's CSD led Milner (8) to posit in 1958 that migraine scotomas are the conscious experience of CSD. In 2001, Hadjikhani et al (9) found that using magnetic resonance imaging (MRI), the average velocity of blood oxygenation level-dependent (BOLD) sig-nal migration on extrastriate cortex during classic migraine (without teichopsia) to be 3.5 ± 1.1 mm/min. Tempering the assertion that BOLD signal change is a surrogate for CSD is the absence of any recording of electroencephalo-graphic changes of CSD in migraine patients (10), and the uncertainty whether BOLD signal strength is due to excit-atory or inhibitory neurophysiological mechanisms (11). LESSON 2: MINIFICATION/MAGNIFICATION Airy observed that the formed elements (teeth) of his teichopsia were small and fine near the point of visual fixation ("sight-point") and "large-toothed towards the periphery of the field." A plausible explanation for minification in the center of the visual field would await Holmes' (6) wartime evidence of "cortical magnification" of the central visual field. In 1991, Horton and Hoyt (12) found that "55% of the surface area of primary visual cortex is devoted to the repre-sentation of the central 10° of vision." Such cortical magnifi-cation would presuppose that in the case of 2 identically sized patches of visual cortex undergoing teichopsia-producing migrainous "discharges," the size of the visual elements would seem smaller when generated by the patch of cortex at the occipital pole and larger when generated by the patch sub-serving peripheral field in anterior calcarine cortex. This was borne out by Brindley and Lewin (13) who demonstrated variability of phosphene size produced by electrical stimula-tion with 0.8 · 0.9 mm platinum electrodes applied to cortex of a blind glaucoma patient. A phosphene produced within 10° of fixation seemed as a "very small spot of white light" and phosphenes elicited more than 20° from fixation were larger ("clouds"). Other scientist migraineurs including Lashley (5), Grusser (14), and Airy (2) also documented that the visual elements of migraine aura seem larger with increasing distance from the fixation point of vision. In addition, the perceived velocity of teichopsia is not uniform as observed by Airy (1), but rather seems to migrate more slowly because the wave of CSD, which is propagated at a constant rate, traverses the region of cortical magnifi-cation subserving central vision, and seems to move more quickly in the peripheral field of vision as CSD passes across regions of cortical minification (15). LESSON 3: COLOR IS DISSOCIABLE FROM TEICHOPSIA Although Airy's fully developed teichopsia was "splendid with large gleams of blue and red and green," color was not an invariable accompaniment, and Airy devotes nearly a page to the "total absence of color" during the teichopsia experienced by Sir Charles Wheatstone, a famed British scientist and inventor. In an effort to explore the variable relationship of color to teichopsia, I asked my patients with classic or acephalgic migraine if their visual aura seemed in color or black and white. On showing Airy's published drawing to 100 consecutive migraineurs with visual aura (24 men, mean age 58.5 years; 76 women, mean age 53.8 years), it was immediately recognized by 48 patients. Twenty-six patients experienced colored teichopsia, and 22 recalled teichopsia devoid of color. Other visual auras lack-ing teichopsic morphology were also colored in 21 patients and achromatopsic in 47 (15 patients had multiple types of visual aura). In my patient cohort, there was a significant association of color with teichopsia (P = 0.136, Fisher exact test). Patient age, gender, or migraine type (classic vs ace-phalgic) was not associated with presence or absence of teichopsia color (unpublished observations, Lepore FE). FIG. 1. Airy's drawing of his own visual aura uses a series of 10 static images to portray the expansion of his left-sided teichopsia throughout its inception, progression, and full development. Each stage of the teichopsia displays "its proper zigzag outline, enriched with tinges of color." Mini-fication of the jagged teeth of the aura at the fixation point ("O") and magnification at greater distances from fixation are consonant with cortical magnification of the central visual field. 312 Lepore: J Neuro-Ophthalmol 2014; 34: 311-314 Historical Note Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Where in the visual pathway could this dissociation of color from form take place? Although wavelength (as distinguished from color) perception could be selectively disrupted at the level of retinal cones (16) or parvocellular layers (17) of the lateral geniculate nucleus (LGN), the center-surround receptive fields of retinal ganglion and LGN neurons would be unlikely sources for the complex geometry of teichopsia. Within the primary visual cortex (V1), dysfunction of wavelength detecting blob cells and unimpaired function of the orientation-selective interblob cells could account for perception of achromatopsic complex forms. Possibly, the distinctive function and metabolism of blob cells using cytochrome oxidase and the presence of double-opponent receptive fields could be subjected to selec-tive metabolic vulnerability during migraine aura (17). The cortical region that "is critical for color vision," is V4 complex located in the fusiform gyrus (18). V4 receives input from blob cells through the "thin stripes" of V2 and transcends "simple" wavelength detection with its capacity for color con-stancy when viewing objects illuminated with light of varying wavelengths. Zeki (18) contends that color constancy relies on the brain's ability to take a ratio of light of any waveband reflected from a surface and its surrounds. Although Zeki notes that it is "impossible to detach color completely from form," patients with lesions of the fusiform gyri (and V4) can accu-rately see forms and shapes, but they lack color. Could migraine-induced selective stimulation of V1 without involve-ment of V4 account for colorless visual auras? Possibly it could, if CSD travels, as posited by Hansen et al (15) "in a noncon-centric manner across different cortical regions" with variable effects on some portions of visual cortex while sparing others. Alternatively, could the association of the teichopsia of Airy with color be due to an anatomic (or physiologic) pairing of the fusiform gyrus and the site(s) of origin of teichopsia? Several supporting lines of anatomical evidence include the abnormally thickened occipital lobe (V3A) cortex and subjacent white matter in migraineurs (19) and the internally generated percept of color in grapheme-color synesthesia, which occurs in the setting of increased ana-tomical connectivity of the fusiform gyrus (20). Blob and interblob cells notwithstanding the dichotomy between orientation-selective and wavelength-sensitive neurons in V1 is not absolute. There are neurons in primate V1 (and V2) that respond to both form and color (21), and these could conceivably underlie migrainous colored teichopsia. WHEN TEICHOPSIA? Hubert Airy pursued a career in British public health as a Medical Inspector with the Local Government Board and published articles on topics in natural history such as bird flight (22), but he never again wrote on the topics of neu-rology or ophthalmology. In his long and enduring contri-bution to clinical neuroscience (1), Airy (23) conceded that "it is impossible at present to do more than guess at the locus morbi," but he nevertheless speculated "that the seat of the affection must lie at some point behind the chiasma." Airy's assignment of the "visual derangement" to the brain and not to the eyes was of fundamental importance. The persistence of the descriptive but inaccurate diagnosis of "optical migraine" reminds the contemporary clinician of Airy's revolutionary conceptualization of migraine as a brain disorder. The quest to localize teichopsia has continued to the present. The distinctive geometric configuration led Richards (24) to posit ocular dominance columns, and Shams and Plant (25) to propose the orientation-specific cells in V1 "interblobs" as neural substrates for teichopsia. However, the hypothesis of V1 as a starting point for visual aura should be questioned after Hadjikhani et al (9) reported initial BOLD signal changes on MRI in V3A in a migraineur with aura of white "TV snow." With his exacting characterization of teichopsia as a "‘Photograph' of a morbid process going on in the brain," Airy ushered an archetypal visual hallucination into the realm of brain biology. This was no mean feat! Seven hun-dred years earlier migraine auras were apprehended as "the Fall of the Angels" by Hildegard of Bingen (26). Airy sur-veyed a world of internally generated visual percepts which, at times, resembled "Alice's Adventures in Wonderland" published 5 years earlier by Lewis Carroll, a migraineur, who had been introduced to Airy's account of teichopsia by Latham's pamphlet "On Nervous or Sick-Headache" (27). In "normal" veridical perception, we know that "no matter how hard you try, you can't see the world in black and white; nor can you see only the left (or right) half of your field of view" (28). These normative rules of percep-tion were broken by Airy's description of teichopsia con-sisting exclusively of internally generated percepts (29). What had begun as an attempt "to collect and record facts" (Airy's italics) about "a morbid affection of the eye-sight" was, in retrospect, a groundbreaking exploration of a little known region of conscious visual experience. Brought forth in the 33rd year of Queen Victoria's reign, Airy's magisterial delineation of teichopsia continues to inform, instruct, and challenge clinicians. REFERENCES 1. Airy H. On a distinct form of transient hemiopsia. Philos Trans R Soc Lond B Biol Sci. 1870;160:247-264. 2. Airy GB. On hemiopsy. Philos Mag. 1865;30:19-21. 3. Plant GT. The fortification spectra of migraine. Br Med J (Clin Res Ed). 1986;293:1613-1617. 4. Hupp SL, Kline LB, Corbett JJ. Visual disturbances of migraine. Surv Ophthalmol. 1989;33:221-236. 5. Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine. Arch Neurol Psychiatry. 1941;46:331-339. 6. Holmes GM. The organization of the visual cortex in man. Proc R Soc Lond B Biol Sci. 1945;132:348-361. 7. Leao AAP. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359-390. 8. Milner PM. Note on a possible correspondence between the scotomas of migraine and spreading depression of Leao. Electroencephalogr Clin Neurophysiol. 1958;10:705. Lepore: J Neuro-Ophthalmol 2014; 34: 311-314 313 Historical Note Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 9. Hadjikhani N, del Rio MS, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Sorensen AG, Moskowitz MA. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692. 10. Charles A. Advances in the basic and clinical science of migraine. Ann Neurol. 2009;65:491-498. 11. Smith DF. Cognitive brain mapping for better or worse. Perspect Biol Med. 2010;53:321-329. 12. Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991;109:816-824. 13. Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196: 479-493. 14. Grusser OJ. Migraine phosphenes and the retino-cortical magnification factor. Vision Res. 1995;35:1125-1134. 15. Hansen JM, Baca SM, VanValkenburgh P, Charles A. Distinctive anatomical and physiological features of migraine aura revealed by 18 years of recording. Brain. 2013;136:3589-3595. 16. Conway BR. Color vision, cones, and color-coding in the cortex. Neuroscientist. 2009;15:274-290. 17. Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740-749. 18. Zeki S. The Ferrier lecture 1995. Behind the seen: functional specialization of the brain in space and time. Philos Trans R Soc Lond B Biol Sci. 2005;360:1145-1183. 19. Granziera C, DaSilva A, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006;3:e402. 20. Hubbard EM. A real red-letter day. Nat Neurosci. 2007;10:671-672. 21. Friedman HS, Zhou H, von der Heydt R. The coding of uniform color figures in monkey visual cortex. J Physiol. 2003;548:593-613. 22. Eadie MJ. Hubert Airy, contemporary men of science and the migraine aura. J R Coll Physicians Edinb. 2009;39:263-267. 23. Airy H. On a distinct form of transient hemiopsia. Proc R Soc Lond B Biol Sci. 1869;18:212-216. 24. Richards W. The fortification illusions of migraines. Sci Am. 1971;224:88-96. 25. Shams PN, Plant GT. Migraine-like visual aura due to focal cerebral lesions: case series and review. Surv Ophthalmol. 2011;56:135-161. 26. Singer C. The scientific views and visions of Saint Hildegard (1098-1180). In: Singer C, eds. Studies in the History and Method of Sciences. Oxford, United Kingdom: Oxford University Press, 1917:1-55. 27. Weatherall MW. The migraine theories of Liveing and Latham: a reappraisal. Brain. 2012;135:2560-2568. 28. Koch C. Consciousness: Confessions of a Romantic Reductionist. Cambridge, United Kingdom: MIT Press, 2012:125. 29. Lepore FE. Effects of visual pathway lesions on the visual aura of migraine. Cephalalgia. 2009;29:430-435. 314 Lepore: J Neuro-Ophthalmol 2014; 34: 311-314 Historical Note Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |