| OCR Text |
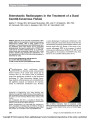
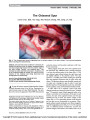
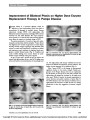
Show Improvement of Bilateral Ptosis on Higher Dose Enzyme Replacement Therapy in Pompe Disease Pompe disease is a lysosomal disorder caused by deficiency of acid a-glucosidase (GAA) that leads to accumulation of glycogen in multiple tissues. Enzyme replacement therapy (ERT) with alglucosidase alfa (Myozyme;Genzyme Corporation, Cambridge, MA) is the first treatment for this lethal disorder. We report improve-ment of ptosis in a 17-year-old boy with nonclassic infantile-onset disease in response to increased dosage of ERT. The patient presented at 6 months of age with cardio-myopathy and proximal skeletal myopathy. Pompe disease was diagnosed at 9 months of age based on muscle biopsy showing vacuolar storage of glycogen and decreased GAA activity in muscle and lymphocytes. In addition to skeletal and respiratory muscle weakness, he displayed slowly pro-gressive, variable bilateral ptosis (Fig. 1). Pupillary and ocular motility findings were normal. Results of acetyl-choline receptor antibody testing were negative. At age 13 years and 4 months of age, the patient was treated with alglucosidase alfa at a dose of 20 mg/kg every other week based on recommendations in the package insert (1). The alglucosidase alfa therapy stabilized but did not improve his skeletal and respiratory muscle weakness. The degree of ptosis appeared to be unaffected (Fig. 2). After 2 years of ERT at this dosage, he demonstrated significant decline in his muscle strength. Besides pro-gression of the Pompe disease, there was no explanation for this decline. At the age of 16 years and 8 months, the alglucosidase alfa dosage was increased to 40 mg/kg every other week. Within 6 months, he showed improvement in muscle function and partial resolution of ptosis (Fig. 3). The ERT infusions were well tolerated. Antibody titers to alglucosidase alfa remained ,1:800. He did not develop proteinuria or show any suggestion of immune complex disease. FIG. 1. Our patient with Pompe disease before treatment. At age 14 months (A), 10 years (B), and 12 years (C), ptosis is progressing. FIG. 2. Treatment with 20 mg/kg alglucosidase alfa biweekly has produced no improvement in ptosis at age 13 years (A) and 15 years (B). FIG. 3. Treatment with 40 mg/kg alglucosidase alfa biweekly has produced improvement in ptosis at age 17 years. Yanovitch et al: J Neuro-Ophthalmol 2010; 30: 165-166 165 Clinical Observation Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. This patient illustrates the need for individualization of ERT dosage based on clinical response. Tammy L. Yanovitch, MD Duke Eye Center Duke University Medical Center Durham, North Carolina Robin Casey, MD Alberta Children's Hospital Calgary Alberta, Canada Suhrad G. Banugaria, MBBS Division of Medical Genetics Department of Pediatrics Duke University Medical Center Durham, North Carolina Priya S. Kishnani, MD Division of Medical Genetics Department of Pediatrics Duke University Medical Center Durham, North Carolina P.S.K. has received research/grant support and honoraria from Genzyme Corporation (Cambridge, MA) and is a member of the Pompe Disease and the Gaucher Disease Registry Advisory Board for Genzyme Corporation. R.C. has received research/grant support and honoraria from Genzyme Corporation. rhGAA, in the form of Genzyme's product, Myozyme, has been approved by the U.S. Food and Drug Administration, Health Canada, and the European Union as therapy for Pompe disease.Duke University and inventors for the method of treatment and predecessors of the cell lines used to generate the enzyme (rhGAA, Myozyme) receive royalty payments pursuant to the university's policy on Inventions, Patents and Technology Transfer. REFERENCE 1. Myozyme package insert. Cambridge, MA: Genzyme Corporation; 2006. Clinical Observation 166 Yanovitch et al: J Neuro-Ophthalmol 2010; 30: 165-166 Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. |