| OCR Text |
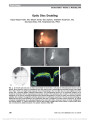
Show Differentiation of Optic Disc Edema From Optic Nerve Head Drusen With Spectral-Domain Optical Coherence Tomography Ozge Sarac, MD, Yelda Y. Tasci, MD, Canan Gurdal, MD, Izzet Can, MD Background: To assess the efficacy of quantitative analysis of the optic nerve head and peripapillary retinal nerve fiber layer (RNFL) with the spectral-domain optical coherence tomography (SD-OCT) in differentiating optic disc edema (ODE) from optic nerve head drusen (ONHD). Methods: Prospective clinical study. Twenty-five eyes of 25 ODE patients (group 1), 25 eyes of 25 ONHD patients (group 2), and 25 eyes of 25 healthy subjects were included. The thickness of the peripapillary RNFL, the thickness of the subretinal hyporeflective space (SHYPS), the area of the SHYPS, the horizontal length of the optic nerve head, and the angle between the temporal RNFL and the optic nerve head (a-angle) were evaluated with SD-OCT. Results: The mean RNFL thickness was significantly greater in group 1 when compared with group 2 and control group (P , 0.001). The receiver operating characteristic curve areas for temporal and nasal RNFL thicknesses in differen-tiating group 1 and group 2 were 0.819 and 0.851, respec-tively (for temporal RNFL thickness .101.5 mm: sensitivity 92%, specificity 65%; for nasal RNFL thickness .74.5 mm: sensitivity 92%, specificity 47%). The mean SHYPS thick-ness, SHYPS area, and degree of the a-angle were greater in group 1 when compared with group 2 (P , 0.05). For the SHYPS thickness .464 mm: 85% sensitivity and 60% spec-ificity; for the SHYPS area .811 mm2: 85% sensitivity and 89% specificity; and for the a-angle .141°: 77% sensitivity and 95% specificity were obtained. Conclusion: The quantitative analysis of the optic nerve head and peripapillary RNFL with SD-OCT can provide useful data in differentiating ODE from ONHD. Journal of Neuro-Ophthalmology 2012;32:207-211 doi: 10.1097/WNO.0b013e318252561b © 2012 by North American Neuro-Ophthalmology Society Optic disc edema (ODE) is usually due to increased intracranial pressure or an optic neuropathy that may necessitate neurologic or systemic evaluation and medical or surgical treatment. Optic nerve head drusen (ONHD) are laminated calcified hyaline bodies that form anterior to the lamina cribrosa of the optic nerve and are not associated with neurologic disease, yet may simulate ODE (1-4). During patient evaluation, differentiation of ODE from ONHD is crucial. Examination techniques commonly performed in the diagnosis of ONHD are B-scan ultraso-nography, fluorescein angiography, and CT (1,5). Applica-tion of optical coherence tomography (OCT), a noninvasive imaging technique that creates images closely resembling histologic sections, recently has achieved increasing popu-larity in differentiating ODE vs ONHD. OCT parameters studied included peripapillary retinal nerve fiber layer (RNFL) thickness (6,7) and direct visualization of the optic nerve head (8). We assessed the efficacy of spectral-domain optical coherence tomography (SD-OCT) in differentiating ODE and ONHD by visualizing the optic nerve head and the peripapillary RNFL. In addition, we sought to identify SD-OCT features that would differentiate ODE from ONHD. METHODS This prospective study was conducted in compliance with the institutional and government review board regulations, informed consent regulations, and the Declaration of Helsinki. Written informed consent was obtained from all patients and control subjects. Twenty-five eyes of 25 ODE patients (11 with papil-ledema, 8 with nonarteritic anterior ischemic optic neurop-athy, and 6 with optic neuritis), 25 eyes of 25 ONHD patients, and 25 eyes of 25 normal subjects were recruited from the Department of Neuro-Ophthalmology of Yildirim Beyazit University, Ataturk Hospital, from December 2009 Yildirim Beyazit University, Ataturk Hospital (OS, YYT, CG, IC), Ophthalmology Department, Bilkent, Ankara, Turkey. The authors report no conflicts of interest. Address correspondence to Ozge Sarac, MD, Meksika Caddesi, Defne Sitesi, 2. Blok, Daire no. 3, Umitkoy, Ankara, 06800, Turkey; E-mail: osarac2002@yahoo.com Sarac et al: J Neuro-Ophthalmol 2012; 32: 207-211 207 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. to April 2011. Patients with ODE formed group 1, and patients with ONHD formed group 2. The degree of ODE was variable from subtle to severe. In bilateral asymmetric cases, only the more edematous optic disc was evaluated, and in bilateral symmetric cases, only the right eye was included for study. Those excluded were patients younger than 7 years or older than 70 years and individuals with high hyperopia (greater than +7.00 diopters [D]) or high myopia (greater than 26.00 D). All patients underwent complete ophthalmologic exami-nation, including visual acuity testing, slit-lamp examination, dilated funduscopy, color fundus photography, autofluor-escence imaging, and ocular echography. Supplemental testing included visual fields and fluorescein angiography. Patients suspected of having a neurologic disorder underwent neurologic examination, brain CT imaging, and cerebrospi-nal fluid analysis. If the diagnosis of ONHD could not be made with funduscopy, it was established by fulfilling at least 2 of the following 4 criteria: autofluorescence on fundus photography, calcification on B-scan ultrasonography or CT, and normal opening pressure on lumbar puncture. Patients with ODE who were included in the study had documented resolution of ODE during the follow-up period. Patients were evaluated with SD-OCT (RTVue, soft-ware version 2.7; Optovue, Inc, Fremont, CA) imaging the optic disc and the peripapillary RNFL. This instrument takes 26,000 A-scans per second, with a frame rate of 256 to 4,096 A-scans per frame. It has a depth resolution of 5 mm and a transverse resolution of 15 mm. The scan range is 2-2.3 mm in depth and 2-12 mm in the transverse plane. The scan beam wavelength is 840 ± 10 nm (9). All OCT measurements were performed by a single examiner (Y.Y.T.). Each participant was instructed to fixate on an external target positioned in the primary position. Multiple horizontal and vertical scans centered 3.45 mm diameter (radius, 1.73 mm) on the optic disc were performed. The configurations of the optic nerve head and retinal layers around the optic nerve head and the average peripapillary RNFL thickness of the superior, inferior, nasal, and temporal quadrants in group 1, group 2, and the control groups were evaluated. The hypore-flective space located between the sensory retina and the retinal pigment epithelium and choriocapillaris complex, designated as the subretinal hyporeflective space (SHYPS), was determined in groups 1 and 2 (10). With the caliper tool provided by the SD-OCT, the thickness (Fig. 1A) and area (Fig. 1B) of the SHYPS were measured. The thickness of the SHYPS was measured from the highest point in each patient. The angle between the retinal pigment epithelium and the outer nuclear layer at the optic nerve head margin, termed the a-angle (Fig. 1C), was measured manually with a caliper tool from the computer screen in the section where the optic nerve head had the highest configuration. Finally, the horizontal length of the optic nerve was determined (Fig. 1D). Statistical analysis was performed using the Statistical Package for Social Sciences software (version 16.0; SPSS, Inc, Chicago, IL). The significance of the difference in the RNLF thickness and optic nerve head parameters were assessed by the analysis of variance test between the study groups and the control group. Differences were considered statistically significant at P # 0.05. FIG. 1. SD-OCT in a patient with ODE. (A) thickness (arrow) of the SHYPS, (B) area of SHYPS, (C) a-angle: the angle between the RNFL and the optic nerve head margin, (D) horizontal length of the optic nerve head. SHYPS, subretinal hyporeflective space. 208 Sarac et al: J Neuro-Ophthalmol 2012; 32: 207-211 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. RESULTS Group 1 was composed of 17 women (68%) and 8 men (32%), and group 2 included 15 women (60%) and 10 men (40%). The average age in group 1 was 38.13 ± 18.84 years (range, 19-61 years) and that in group 2 was 29.29 ± 15.58 years (range, 7-55 years). The control group con-sisted of 15 women (60%) and 10 men (40%), with an average age of 32.53 ± 15.05 years (range, 18-52 years). The mean RNFL thickness was significantly greater in group 1 when compared with group 2 and the control group (P , 0.001; Table 1). No statistically significant difference between group 2 and the control group was seen (P = 0.320; Table 1). While differentiating groups 1 and 2, the receiver operating characteristic (ROC) curve areas were calculated in each quadrant for the RNFL thickness. The ROC curve area for temporal RNFL thickness was 0.819. When the cutoff point for temporal RNFL thickness was set at 101.5 mm, 92% sensitivity and 65% specificity were obtained. The area under the ROC curve for nasal RNFL thickness was 0.851 (for RNFL thickness .74.5 mm: sen-sitivity 92%, specificity 47%). The mean thickness of the highest point of the SHYPS was 582.27 ± 208.16 mm in group 1 and 456.77 ± 112.07 mm in group 2 (P = 0.04). When the cutoff point for the thickness of the highest point of the SHYPS was set at 464 mm, 85% sensitivity and 60% specificity were obtained. The mean area of the SHYPS was 1,110 ± 210 mm2 in group 1 and it was decreased to 620 ± 120 mm2 in group 2 (P = 0.008). The area under the ROC curve for this parameter was 0.851 (for area .811 mm2: sensitivity 85%, specificity 89%). The mean degree of the a-angle was 145.77 ± 6.34° in group 1 compared with 131.18 ± 11.89° in group 2 (P , 0.001). The area under the ROC curve for this parameter was 0.896 (for angle .141°: sensitivity 77%, specificity 95%). The mean horizontal length of the optic nerve head was 2,530 ± 830 mm in group 1. The value was 1,920 ± 241 mm in group 2 and 1,530 ± 200 mm in the control subjects. The differences between groups 1 and 2 (P = 0.007) and group 1 and the control group (P , 0.01) were statistically significant. The mean horizontal length of the optic nerve head was positively correlated with the mean RNFL thick-ness (r = 0.557, P , 0.001), the mean SHYPS thickness (r = 0.757, P , 0.001), and the mean area of the SHYPS (r = 0.927, P , 0.001). DISCUSSION ONHD are a common, benign, congenital anomaly of the optic nerve, which rarely lead to decreased visual acuity (1,11). It is thought that the formation of ONHD is caused by axoplasmic transport alteration and axonal degeneration in the presence of a small scleral canal (12). In affected patients, the configuration of the optic nerve head is vari-able, and the drusen may be visible on the disc surface or buried within the disc. It is buried ONHD that may simulate ODE and lead to diagnostic uncertainty. Techniques to differentiate these 2 conditions include funduscopy, optic disc autofluorescence, fluorescein angiography, B-scan ultrasonography, and CT scanning. B-scan ultrasonography has been shown to be superior to autofluorescence and CT (13). In recent years, there have been a number of reports evaluating OCT to distinguish between ODE and ONHD, focused primarily on measurements of the peripapillary RNFL thickness (6,8,10,14). RNFL thickness, especially in the nasal quad-rant, has been shown to be decreased in ONHD when compared with ODE (8). In some patients with ONHD, photoreceptor changes also have been documented (15). Using OCT, Savini et al (10) identified the SHYPS, a hyporeflective space located between the sensory retina and the retinal pigment epithelium and choriocapillaris in ODE patients. Johnson et al (7) found a decrease in the mean SHYPS thickness in ONHD patients compared with those with ODE. They considered the extravasated fluid from the optic nerve head, percolating into and elevating the subretinal space, as the most plausible cause for the increased SHYPS thickness. These investigators character-ized the OCT appearance of ODE as an elevated optic nerve head with a smooth internal contour and a SHYPS TABLE 1. RNFL thickness in patients with ODE, ONHD, and controls ODE (m) ONHD P (ODE vs ONHD) Controls P (ONHD vs Controls) P (ODE vs Controls) Temporal 132.87 ± 25.43 102.24 ± 31.76 0.012 136.20 ± 28.40 0.005 0.946 Superotemporal 182.87 ± 50.85 165.77 ± 56.6 0.556 138.6 ± 24.6 0.236 0.032 Inferotemporal 187.4 ± 50.67 183.3 ± 31.26 0.957 101.2 ± 39.8 0.001 0.001 Nasal 133.2 ± 52.68 87.12 ± 28.8 0.003 122.73 ± 25.37 0.026 0.724 Superonasal 177.47 ± 51.4 150.18 ± 54.23 0.263 96.47 ± 37.84 0.009 0.001 Inferonasal 181.47 ± 56.39 148.65 ± 38.3 0.069 149.47 ± 18.25 0.998 0.090 Average 156.87 ± 31.59 128.24 ± 29.85 0.001 114.60 ± 12.98 0.320 0.001 ODE, optic disc edema; ONHD, optic nerve head drusen; RNFL, retinal nerve fiber layer Sarac et al: J Neuro-Ophthalmol 2012; 32: 207-211 209 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. thickness under the optic nerve head with a gradient taper away from the disc. A "lumpy bumpy" internal optic nerve contour and a more abrupt taper of the SHYPS (Fig. 2) were suggestive of ONHD (7). We differentiated ODE from ONHD using quantitative measures obtained with SD-OCT: peripapillary RNFL thickness, SHYPS thickness, area of the SHYPS, and degree of the a-angle. Measuring RNFL thickness with SD-OCT, differentia-tion of ODE from ONHD ranged in sensitivity from 77% to 92% (temporal and nasal RNFL thicknesses greater than 101.5 and 74.5 mm, respectively) and specificity from 47% to 95% (a-angle greater than 141°). The mean RNFL thickness was higher in ODE patients in all quadrants when compared with that of ONHD patients. When we evalu-ated the respective peripapillary RNFL thicknesses, the ROC curve showed that the temporal and nasal RNFL thicknesses were the most important parameters for differ-entiating these 2 disorders. The temporal RNFL thickness greater than 101.5 mm had 92% sensitivity and 65% specificity. The nasal RNFL thickness greater than 74.5 mm had 92% sensitivity and 47% specificity. Johnson et al (7) reported 80% specificity and 70% sen-sitivity for the temporal RNFL thickness greater than 97 mm and nasal RNFL thickness greater than 86 mm for the differentiation of ONHD from ODE. Lee et al (8) investigated the differentiation of ONHD from ODE with SD-OCT and demonstrated the nasal RNFL thickness as the most important factor. They detected 80.0% sensitivity and 88.9% specificity for nasal RNFL thickness greater than 78.0 mm. Measuring the thickness of the SHYPS also helped distinguish between ODE and ONHD, being greater in ODE patients. SHYPS thickness greater than 464 mm had 85% sensitivity and 60% specificity. Johnson et al (7) measured the SHYPS thickness at radii of 0.75, 1.5, and 2 mm in ONHD and ODE patients and reported the sensitivity and specificity for the 2-mm radius for SHYPS thickness as 70% and 90%, respec-tively (for SHYPS thickness .169 mm). Their cutoff point for this parameter was thinner than that of 464 mm in our study. The area of the SHYPS and the a-angle were signifi-cantly higher in ODE patients than in patients with ONHD. These 2 parameters had the highest specificity values. The area of the SHYPS greater than 811 mm2 had 89% specificity and proved to be a better method of distinguishing ODE from ONHD than the thickness of the SHYPS, both in our study and previous reports (7,8). Measurement of the a-angle was also a highly predictive parameter as a measurement greater than 141° had a 95% specificity. While the horizontal length of the optic nerve head in ODE patients was significantly greater than that in patients with ONHD and control subjects, it did not distinguish between patients with ONHD and control subjects. Our findings are in agreement with those of Floyd et al (16) and do not support the hypothesis that ONHD patients have a small scleral canal that causes a crowding effect on axonal transport or neural development. We recognize the limitations of our study. First, the sample sizes were small with wide age range in all groups. Second, measurement of the a-angle was performed man-ually. Third, we did not evaluate the qualitative parameters of the optic nerve head and the peripapillary RNFL. Despite these limitations, we believe that our study presents prom-ising findings in support of the use of SD-OCT in differ-entiating ODE from ONHD. Further studies with large patient groups are needed to validate the proposed cutoff values that we obtained. REFERENCES 1. Auw-Haedrich C, Staubach F, Witschel H. Optic disc drusen. Surv Ophthalmol. 2002;47:515-532. 2. Giarelli L, Ravalico G, Savino S, Grandi A. Optic nerve head drusen: histopathological considerations-clinical features. Metab Pediatr Syst Ophthalmol. 1990;13:88-91. 3. Wilkins JM, Pomeranz HD. Visual manifestations of visible and buried optic disc drusen. J Neuroophthalmol. 2004;24:125-129. 4. Friedman AH, Gartner S, Modi SS. Drusen of the optic disc. A retrospective study in cadaver eyes. Br J Ophthalmol. 1975 413-421. 5. Pineles SL, Arnold AC. Fluorescein angiographic identification of optic disc drusen with and without optic disc edema. J Neuroophthalmol. 2012;32:17-22. 6. Karam EZ, Hedges TR. Optical coherence tomography of the retinal nerve fiber layer in mild papilloedema and pseudopapilloedema. Br J Ophthalmol. 2005;89: 294-298. 7. Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127:45-49. 8. Lee MK, Woo SJ, Hwang JM. Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:971-977. 9. Alam S, Zawadzki RJ, Choi S, Gerth C, Park SS, Morse L, Werner JS. Clinical application of rapid serial Fourier domain optical coherence tomography for macular imaging. Ophthalmology. 2006;113:1425-1431. 10. Savini G, Bellusci C, Carbonelli M, Zanini M, Carelli V, Sadun AA, Barboni P. Detection and quantification of retinal FIG. 2. SD-OCT in a patient with ONHD. Note the lumpy-bumpy internal contour of the optic nerve head and an abrupt decline in the border of the SHYPS. 210 Sarac et al: J Neuro-Ophthalmol 2012; 32: 207-211 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. nerve fiber layer thickness in optic disc edema using stratus OCT. Arch Ophthalmol. 2006;124:1111-1117. 11. Lee AG, Zimmerman MB. The rate of visual field loss in optic nerve head drusen. Am J Ophthalmol. 2005;139:1062-1066. 12. Lam BL, Morais CG Jr, Pasol J. Drusen of the optic disc. Curr Neurol Neurosci Rep. 2008;8:404-408. 13. Kurz-Levin MM, Landau K. A comparison of imaging techniques for diagnosing drusen of the optic nerve head. Arch Ophthalmol. 1999;117:1045-1049. 14. Menke MN, Feke GT, Trempe CL. OCT measurements in patients with optic disc edema. Invest Ophthalmol Vis Sci. 2005;46:3807-3811. 15. Choi SS, Zawadzki RJ, Greiner MA, Werner JS, Keltner JL. Fourier-domain optical coherence tomography and adaptive optics reveal nerve fiber layer loss and photoreceptor changes in a patient with optic nerve drusen. J Neuroophthalmol. 2008;28:120-125. 16. Floyd MS, Katz BJ, Digre KB. Measurement of the scleral canal using optical coherence tomography in patients with optic nerve drusen. Am J Ophthalmol. 2005;139:664-669. Sarac et al: J Neuro-Ophthalmol 2012; 32: 207-211 211 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |