| Title |
Fundamentals of Hazardous Solid Waste Incineration in a Rotary Kiln Environment |
| Creator |
Lighty, JoAnn S.; Silcox, Geoff D.; Britt, Robert ; Owens, Warren D.; Pershing, David W.; Cundy, Vic A. |
| Publisher |
University of Utah |
| Date |
1987 |
| Spatial Coverage |
presented at Palm Springs, California |
| Abstract |
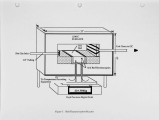
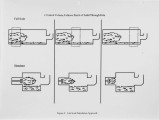
With landfill costs increasing and regulations on landfilling becoming more stringent, alternatives to conventional hazardous waste treatment strategies are becoming more desirable. Incineration is presently a pennanent, proven solution for the disposal of most organic contaminants, but also a costly one, especially in the case of solids which require some auxiliary fuel. The goal of this research is to develop an understanding of the phenomena associated with the evolution of contaminants from solids in the primary combustor of an incineration system. A three fold approach has been used. First, a bench-scale Particle Characterization Reactor was developed to study the transport phenomena on a particle basis, where the controlling processes are mainly intrapanicle. Second, a Bed Characterization Reactor was built to examine the controlling transport phenomena within a bed of panicles, where the processes are primarily interparticle. The results of these studies can be applied to any primary combustor. Finally a pilot-scale rotary kiln was developed to study the evolution of contaminants from solids within a realistic temperature and rotation environment. This paper describes results obtained in a study using a commercial sorbent contaminated with toluene. The data are from the Particle Characterization Reactor and the Rotary-Kiln Simulator. The results show that the method of contamination and charge size do not have a large effect on desorption, while temperature and contaminant concentration are imponant parameters in the evolution of contaminants in a rotary kiln. Preliminary modeling efforts for the kiln are also discussed. |
| Type |
Text |
| Format |
application/pdf |
| Language |
eng |
| Rights |
This material may be protected by copyright. Permission required for use in any form. For further information please contact the American Flame Research Committee. |
| Conversion Specifications |
Original scanned with Canon EOS-1Ds Mark II, 16.7 megapixel digital camera and saved as 400 ppi uncompressed TIFF, 16 bit depth. |
| Scanning Technician |
Cliodhna Davis |
| ARK |
ark:/87278/s6s75jw7 |
| Setname |
uu_afrc |
| ID |
4134 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6s75jw7 |