| Title |
Liquid Injection Incineration an Ultimate Solution to Hazardous Waste Combustion |
| Creator |
Santoleri, Joseph J. |
| Publisher |
University of Utah |
| Date |
1984 |
| Spatial Coverage |
presented at Tulsa, Oklahoma |
| Abstract |
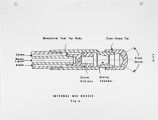
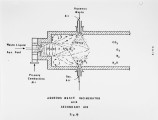
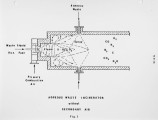
Many liquid wastes considered toxic and hazardous have found their way into landfills, streams and disposal sites. These solutions have only moved the problem from the generator's site to a new location, resulting in our present environmental problems and SUPERFUND. Certain hydrocarbons can only be rendered harmless by converting all carbons to carbon dioxide and hydrogen to water vapor. This can be accomplished in a high temperature reactor or oxidizer. This discussion will cover the physical data needed to properly design a thermal oxidation system for hazardous liquids. This will include information needed to insure proper transport of the material from the holding tanks to the atomizer, proper dispersion of the material into the high temperature flame zone, furnace design to insure adequate mixing, vaporization and oxidation of the organics to final inert products of combustion. Liquids generated from processes associated with chemical, petrochemical, agricultural and pharmaceutical processes will be covered. These will range from the highly aqueous (95% E<0) to the halogenated organics (chlorinated hydrocarbons); heating values ranging from zero Btu/lb to 19,000 Btu/lb; viscosities from 1 cp at 60°F to 1000 cp at 450°F. These characteristics tend to create all types of problems in an adequate disposal system. Our experience in test burns of these materials, as well as the design and supply of operating units, will be covered in this paper. |
| Type |
Text |
| Format |
application/pdf |
| Language |
eng |
| Rights |
This material may be protected by copyright. Permission required for use in any form. For further information please contact the American Flame Research Committee. |
| Conversion Specifications |
Original scanned with Canon EOS-1Ds Mark II, 16.7 megapixel digital camera and saved as 400 ppi uncompressed TIFF, 16 bit depth. |
| Scanning Technician |
Cliodhna Davis |
| ARK |
ark:/87278/s6rx9fmh |
| Setname |
uu_afrc |
| ID |
1863 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6rx9fmh |