| Description |
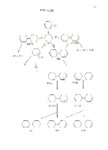
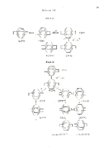
The chemical behavior of some nonaromatic radical anions was investigated by optimizing their lifetimes using inverse metalammonia reduction techniques. By varying reaction conditions such as substrate concentration, proton source, reaction temperature, counter ion, and solvent it was possible to show that the radical anion of 1,3-cyclohexadiene can undergo the same reactions observed for the more stable aromatic radical anions, in addition to the normally observed reduction to cyclohexene. The identification of a minor product from the inverse sodiumamonia reduction of 1,3-cyclohexadiene, lead to the discovery that, under proper conditions, nonaromatic radicals and anions could undergo a coupling reaction, presumably via an intermediate radical anion. A series of experiments was run using various saturated and unsaturated radicals and anions to determine the scope of the reaction. Results indicated that either the radical or the anion must have a site of unsaturation sufficiently close to the incipient bond as to impart some stability to the intermediate radical anion. A study of the reduction of nortricyclanone under various conditions showed that the cyclopropyl ring opening occurs via the radical anion. Conditions which maximized the lifetime of the radical anion produced almost complete ring opened products, while conditions which minimized the lifetime resulted in little or no ring opening. A study of the Birch reduction products of [2.2Jparacyclophane showed that two products were formed. The major product was identified as the tetrahydroparacyclophane, and was assigned the structure based upon its ultraviolet and 13C NMR spectra, and some chemical reactions. The minor product was identifed as the dihydroparacyclophane. Some compounds which possessed nonconjugated, but spacially close, sites of unsaturation were studied electrochemically and chemically to determine the effectiveness of homoconjugation in promoting reduction by electron addition. The results for each compound were compared to those for the saturated analogue. |