| Title |
Pachymeningitis with Mulitple Cranial Neuropathies and Unilateral Optic Neuropathy Secondary to Pseudomonas aeruginosa |
| Creator |
Girkin, CA; Perry, JD; Miller, NR; Reich, SG |
| Affiliation |
Department of Ophthalmology, The Johns Hopkins Medical Institutions, Baltimore, Maryland, USA. |
| Abstract |
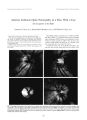
Hypertrophic cranial pachymeningitis is a rare disorder that frequently presents with multiple cranial neuropathies. This disorder, which is characterized by thickening and infiltration of the cranial dura, can result from a variety of inflammatory and infectious conditions. A patient with hypertrophic cranial pachymeningitis is described in whom meningeal biopsy and bacterial cultures of the biopsy specimen revealed Pseudomonas aeruginosa. The authors believe this to be the first documented case of pachymeningitis secondary to this organism. |
| Subject |
Ceftazidime/therapeutic use; Cranial Nerve Diseases/diagnosis; Cranial Nerve Diseases/drug therapy; Cranial Nerve Diseases/microbiology; Drug Therapy, Combination/therapeutic use; Humans; Hypertrophy; Magnetic Resonance Imaging; Male; Meninges/microbiology; Meninges/pathology; Meningitis, Bacterial/diagnosis; Meningitis, Bacterial/drug therapy; Meningitis, Bacterial/microbiology; Middle Older people; Optic Nerve Diseases/diagnosis; Optic Nerve Diseases/drug therapy; Optic Nerve Diseases/microbiology; Paralysis/diagnosis; Paralysis/drug therapy; Paralysis/microbiology; Pseudomonas Infections/diagnosis; Pseudomonas Infections/drug therapy; Pseudomonas Infections/microbiology; Pseudomonas aeruginosa/isolation & purification; Tobramycin/therapeutic use; Tomography, X-Ray Computed |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
224937 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6nc968p/224937 |