| OCR Text |
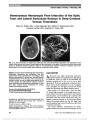
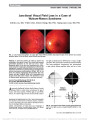
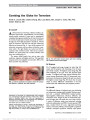
Show Combing the Globe for Terrorism Norah S. Lincoff, MD, Charles Chung, MD, Lucia Balos, MD, Joseph C. Corbo, MD, PhD, Aseem Sharma, MD Dr Lincoff: An 83-year-old man with Type 2 diabetes mellitus, sys-temic hypertension, atrial fibrillation, and successfully treated prostate carcinoma (6 years prior to presentation) complained of progressive painful loss of vision in his left eye for 2 weeks. Examination revealed visual acuity of 20/20 in the right eye and no light perception in the left eye. The left pupil was amaurotic, and there was mild left conjunctival injection and chemosis (Fig. 1), 1 mm of left proptosis, full extraocular movements, and no evidence of anterior or pos-terior uveitis. The right fundus was normal and left was consistent with combined central retinal artery occlusion (CRAO) and central retinal vein occlusion (CRVO) with optic nerve swelling (Fig. 2). The patient denied temple, jaw, and ear pain, scalp tenderness, migratory arthralgias, and fever. The superficial temporal arteries were palpable and nontender. MRI of the brain and orbits was performed. Dr Sharma: The T1-weighted axial image through the orbits (Fig. 3A) reveals mild left proptosis with thickening of the left optic nerve-sheath complex. T2-weighted coronal image (Fig. 3B) shows thickening of the optic nerve with increased signal intensity. T1-weighted axial image obtained following intra-venous contrast administration (Fig. 3C) demonstrates tram-track pattern of enhancement along the left optic nerve sheath. T2-weighted coronal image (Fig. 3D) shows that the optic chiasm is normal. Differential considerations at this time would include both inflammatory and neoplastic processes. Dr Lincoff: The differential diagnosis of unilateral optic nerve thickening and enhancement causing painful unilateral blindness associ-ated with a combined CRVO/CRAO included embolic, hypercoagulable, inflammatory, infectious, infiltrative, and compressive causes. Despite the lack of constitutional symp-toms and signs, giant cell arteritis also was a major concern. A variety of laboratory studies were performed, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) assay, complete blood cell count, angiotensin-converting enzyme, antinuclear antibody, antineutrophilic cytoplasmic antibody, neutrophil cytoplasmic antibody, rapid plasma FIG. 1. External appearance of patient shows left conjunc-tival injection, chemosis, and mild proptosis. FIG. 2. The left fundus shows severe optic disc swelling with scattered retinal hemorrhages. Departments of Neurology and Ophthalmology (NSL), Radiology (CC), and Pathology (LB), State University of New York at Buffalo School of Medicine, Buffalo, New York; and Departments of Pathology and Immunology ( JCC) and Radiology (AS), Washington University School of Medicine, St Louis, Missouri. The authors report no conflicts of interest. Address correspondence to Norah S. Lincoff, MD, Department of Neurology and Ophthalmology, The Jacobs Neurological Institute, State University of New York at Buffalo School of Medicine, 100 High Street, Buffalo, NY 14203; E-mail: lincoff@buffalo.edu 82 Lincoff et al: J Neuro-Ophthalmol 2012; 32: 82-85 Clinical-Pathological Case Study Section Editor: Neil R. Miller, MD Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. reagin, and prostate-specific antigen levels. The results of these studies were negative or normal. Specifically, the ESR was 2 mm/h, and the CRP was 0.85 mg/L (normal, ,1 mg/L). A chest radiograph was normal. Cerebrospinal fluid analysis revealed no cells but a mildly elevated protein of 65 mg/dL (normal, #45 mg/dL). Whole-body positron emis-sion tomographic scan revealed only an inflammatory thy-roid nodule confirmed by biopsy. A temporal artery biopsy was normal. The retinal hemorrhages and disc swelling in the left eye resolved over the next 5 months, but the patient developed rubeotic glaucoma treated with 2 intravitreal injections of bevacizumab. In addition, he was given oral prednisone beginning with a dose of 1 mg/kg/d, resulting in resolution of his left periorbital pain within 4 weeks. An optic nerve biopsy was recommended but was not performed because the patient was taking Coumadin for atrial fibrillation. A repeat MRI was performed 9 months after the onset of symptoms. Dr Sharma: The follow-up MRI demonstrates progression of the initial abnormality, which is manifest as thickening and hyper-intensity of the intracranial portion of the left optic nerve, the optic chiasm, and the left optic tract, as seen on axial and sagittal FLAIR images (Fig. 4A, C). T1-weighted cor-onal image after intravenous injection of contrast (Fig. 4B) shows thickening and enhancement of the chiasm. In addi-tion, axial FLAIR image (Fig. 4A) demonstrates abnormal signal in the left medial temporal lobe, suggesting extension along the optic radiations. The pattern of progression sug-gests an infiltrative process. FIG. 3. Magnetic resonance findings at presentation. A. T1 axial image shows enlargement of the left optic nerve. B. T2 coronal scan confirms enlargement of the left optic nerve with increased signal (arrow). C. Contrast-enhanced T1 axial image reveals enhancement of left optic nerve sheath. D. T2 coronal scan demonstrates normal appearance of the optic chiasm (arrow). Lincoff et al: J Neuro-Ophthalmol 2012; 32: 82-85 83 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Dr Lincoff: Following temporary cessation of Coumadin, a craniotomy was performed. The intracranial segment of the left optic nerve and the optic chiasm appeared abnormally large, red, and hypervascular. On cross section, the tumor was white with a hemorrhagic center (Fig. 5A, B). A large segment of the intracranial portion of the left optic nerve was resected. Dr Corbo: The specimen shows a markedly cellular, diffuse, astrocytic neoplasm with mitotic activity, incipient necrosis, and vascular proliferation (Fig. 6). The tumor cells were positive for the astrocytic marker glial fibrillary acidic protein, and an immunostain for Ki67 revealed a proliferation index of 17%. Thick hyalinized septae were noted throughout the specimen at low power, suggesting infiltration of the optic nerve by the tumor. Pathologic diagnosis: glioblastoma involving the left optic nerve. FIG. 4. MRI obtained 9 months after presentation. A. Axial FLAIR image reveals enlargement and increased signal in the optic chiasm, left intracranial optic nerve, and left temporal lobe (arrow). B. Contrasted T1 coronal view shows enhancement and enlargement of the optic chiasm (arrow). C. Sagittal FLAIR image demonstrates increased signal in and enlargement of the chiasm and left optic tract (arrow). FIG. 5. Intraoperative photographs. Appearance of the left intracranial optic nerve before (A) and after (B) resection. FIG. 6. Biopsy specimen shows a hypercellular neoplasm with abundant mitotic figures (hematoxylin & eosin, · 100). 84 Lincoff et al: J Neuro-Ophthalmol 2012; 32: 82-85 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Dr Lincoff: Although glioblastoma multiforme (GBM) is the most common glial brain tumor in adults (1), GBMs arising within the anterior visual pathway are uncommon, and cases arising within 1 optic nerve are rare. Of 45 cases of adult malignant optic glioma reported in the literature (2-5), only 3 (2-5) were unilateral (4,5). Malignant gliomas of the anterior visual pathway are slightly more common in males (51% vs 49%) and usually occur in the sixth decade of life or later, with a mean age at diagnosis of 54 years. High-grade astrocytic neoplasms are defined by the presence of mitotic activity and are classified pathologically as either anaplastic astrocytomas (World Health Organization [WHO] Grade III) or GBM (WHO Grade IV) (6). Whereas Grade III tumors lack necrosis and vascular proliferation, the presence of one or the other is required for a Grade IV diagnosis. Survival depends on tumor grade, resectability, and the age of the patient, but they prove fatal in the majority of cases; the mean survival from the time of presentation usually is less than 1 year. Standard therapy is resection followed by radiation therapy and chemotherapy, with the alkylating agent temozolomide. Some improvement in survival has been achieved with monoclonal antibodies directed against the tumor and anti-vascular endothelial growth factor agents (2). Because most malignant gliomas of the anterior visual pathway originate in the optic chiasm or distal optic nerve, patients with these lesions usually present with rapidly progressive unilateral or bilateral painless visual loss associ-ated with normal-appearing fundi. When the tumor arises in the proximal optic nerve, severe visual loss associated with a painful CRVO or mixed CRAO/CRVO is the typical presenting sign (1). This mandates neuroimaging to determine if the fundus abnormalities have been caused by infiltration of the optic nerve with secondary vascular compromise and a canalicular compartment syndrome. The ischemic state of the tissues and ensuing necrosis is the cause of the prolonged pain syndrome; once the necrosis is complete, the pain usually subsides. Recognition of this triad of severe orbital or ocular pain, a fundus picture of a CRVO or mixed CRVO/CRAO, and visual loss that may be greater than one might expect from the appearance of the fundus should prompt early diagnosis and treatment of this neoplasm. REFERENCES 1. Hoyt WF, Meshel LG, Lessell S, Schatz NJ, Suckling RD. Malignant optic glioma of adulthood. Brain. 1973;96:121-132. 2. Pruitt A. Update on treatment of primary and secondary central nervous system tumors [syllabus]. NANOS. 2010:259-262. 3. Taylor T, Jaspan T, Milano G, Gregson R, Parker T, Ritzmann T, Benson C, Walker D. Radiological classification of optic pathway gliomas: experience of a modified functional classification system. Br J Radiol. 2008;81:761-766. 4. Millar WS, Tartaglino LM, Sergott RC, Friedman DP, Flanders AE. MR of malignant optic glioma of adulthood. AJNR Am J Neuroradiol. 1995;16:1673-1676. 5. Wabbels B, Demmler A, Seitz J, Woenckhaus M, Bloss HG, Lorenz B. Unilateral adult malignant optic nerve glioma. Graefes Arch Clin Exp Ophthalmol. 2004;242:741-748. 6. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumours of the Central Nervous System. Lyon, France: International Agency for Research on Cancer, 2007. Lincoff et al: J Neuro-Ophthalmol 2012; 32: 82-85 85 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |