Contents | 1 of 374
Page 1
| Title | Rational design of β-Cathenin/B-cell lymphoma 9 inhibitors, efforts to express functional ethylene receptor, and mapping the electrostatic topology of ubiquinol oxidase |
| Publication Type | dissertation |
| School or College | College of Science |
| Department | Chemistry |
| Author | Wisniewski, John Andrew |
| Date | 2019 |
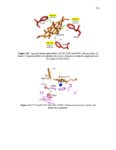
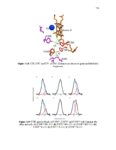
| Description | Chapter 1 focuses on the inhibition of the β-catenin/B-Cell lymphoma 9 protein- protein interaction. This protein-protein interaction is found in Wnt signaling and has been strongly linked to cancer. To inhibit the interaction, molecules were computationally designed, synthesized and tested. A series of 33 final compounds was synthesized, resulting in a lead molecule with an inhibition constant of 5.2 μM. The binding mode of this molecule was validated through the structure activity relationship of the series as well as mutational studies. The lead molecule was shown to exhibit 98-fold selectivity for the β-catenin/B-Cell lymphoma 9 interaction over the β-catenin/E-cadherin interaction, inhibit the Wnt pathway in a cell based assay and inhibit the growth of Wnt/ β-catenin-dependent cancer cells. Chapter 2 describes an attempt to overexpress and purify the ethylene receptor, a membrane protein found in plants critical to all stages of plant life. There are major unanswered questions involving the ethylene receptor, but most important is the nature of the binding interaction between ethylene and the receptor. We intended to characterize the interaction using a variety of spectral techniques, which would require large amounts of protein. Several strategies to express and purify the receptor were pursued. Chapter 3 lays the foundation for using Stark vibrational spectroscopy to more thoroughly understand the mechanism of proton pumping. Stark vibrational probes were installed in the proton pump ubiquinol oxidase. Functional protein containing the probes was successfully expressed and purified. The probes were then utilized to survey the electric field within the proton pump. Changes in electric field were detected in multiple locations and synchronistic results were observed for probes positioned in the same region of the protein. The presented work was highly decoupled and covered a broad area. Completion required efforts in organic synthesis, molecular biology, protein chemistry and spectroscopy. Overall, important foundational achievements were made on several challenging and significant systems. |
| Type | Text |
| Publisher | University of Utah |
| Dissertation Name | Doctor of Philosophy |
| Language | eng |
| Rights Management | © John Andrew Wisniewski |
| Format | application/pdf |
| Format Medium | application/pdf |
| ARK | ark:/87278/s62r9t3d |
| Setname | ir_etd |
| ID | 1715223 |
| Reference URL | https://collections.lib.utah.edu/ark:/87278/s62r9t3d |
Page Metadata
| Title | Page 1 |
| School or College | College of Science |
| Department | Chemistry |
| Description | Chapter 1 focuses on the inhibition of the β-catenin/B-Cell lymphoma 9 proteinprotein interaction. This protein-protein interaction is found in Wnt signaling and has been strongly linked to cancer. To inhibit the interaction, molecules were computationally designed, synthesized and tested. A series of 33 final compounds was synthesized, resulting in a lead molecule with an inhibition constant of 5.2 μM. The binding mode of this molecule was validated through the structure activity relationship of the series as well as mutational studies. The lead molecule was shown to exhibit 98-fold selectivity for the β-catenin/B-Cell lymphoma 9 interaction over the β-catenin/E-cadherin interaction, inhibit the Wnt pathway in a cell based assay and inhibit the growth of Wnt/β-catenin-dependent cancer cells. Chapter 2 describes an attempt to overexpress and purify the ethylene receptor, a membrane protein found in plants critical to all stages of plant life. There are major unanswered questions involving the ethylene receptor, but most important is the nature of the binding interaction between ethylene and the receptor. We intended to characterize the interaction using a variety of spectral techniques, which would require large amounts of protein. Several strategies to express and purify the receptor were pursued. Chapter 3 lays the foundation for using Stark vibrational spectroscopy to more thoroughly understand the mechanism of proton pumping. Stark vibrational probes were installed in the proton pump ubiquinol oxidase. Functional protein containing the probes iv was successfully expressed and purified. The probes were then utilized to survey the electric field within the proton pump. Changes in electric field were detected in multiple locations and synchronistic results were observed for probes positioned in the same region of the protein. The presented work was highly decoupled and covered a broad area. Completion required efforts in organic synthesis, molecular biology, protein chemistry and spectroscopy. Overall, important foundational achievements were made on several challenging and significant systems. |
| Format | application/pdf |
| Setname | ir_etd |
| ID | 1715224 |
| Reference URL | https://collections.lib.utah.edu/ark:/87278/s62r9t3d/1715224 |