| OCR Text |
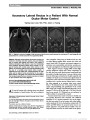
Show Sequential Episodes of Perioperative Ischemic Optic Neuropathy After Hip Surgery Brigid K. Marshall, BS, Manik Goel, MD, Ian F. Pitha, MD, PhD, Gregory P. Van Stavern, MD, Collin M. McClelland, MD Abstract: Perioperative ischemic optic neuropathy (ION) after nonocular surgery is a rare complication leading to permanent and often severe vision loss. Due in part to the low prevalence of this complication, there remains no reliable way to predict which patients will develop ION. We present a patient with sequential episodes of unilateral perioperative ION, both occurring after otherwise uncompli-cated hip operations. Patients and physicians should be aware that perioperative ION after one surgery may increase the risk of ION after subsequent surgeries. Journal of Neuro-Ophthalmology 2014;34:165-168 doi: 10.1097/WNO.0000000000000093 © 2014 by North American Neuro-Ophthalmology Society Perioperative vision loss is a rare surgical complication most commonly due to ischemic optic neuropathy (1). Perioperative anterior ischemic optic neuropathy (AION) most commonly occurs after cardiac procedures and is thought to arise from hypoperfusion of the posterior ciliary arteries supplying the optic nerve head (2). Previously reported cases of perioperative AION document acute uni-lateral or bilateral eye involvement after a single surgical procedure (2-4). Our patient experienced sequential epi-sodes of perioperative ischemic optic neuropathy (ION) in each eye after otherwise uncomplicated hip operations. CASE REPORT A 50-year-old man, known to have tested positively for HIV, developed vision loss in the right eye after hip surgery. Three days previously, he underwent a total left hip explantation with debridement of an infected prosthetic hip. Two days later, he had repeat debridement of the left hip with placement of an antibiotic spacer. After the second operation, he noted sudden onset of painless vision loss in the superior visual field of the right eye. On examination, visual acuity was 20/50, right eye and 20/30, left eye. Confrontation visual fields showed a supe-rior visual field defect in the right eye and a right relative afferent pupillary defect (RAPD). Funduscopic examination revealed right optic disc edema and superotemporal pallor of the left disc (Fig. 1). No evidence of HIV retinopathy was present in either eye. Noncontrast orbital computed tomography was normal. Two days later, vision in the right eye decreased to no light perception with increased optic nerve swelling. The patient was discharged home with a diagnosis of perioperative right AION. Both surgical procedures preceding vision loss were performed in the lateral decubitus position. The first lasted approximately 4.25 hours with an estimated blood loss (EBL) of 3,200 mL. The patient received 3,700 mL crystalloid, 1,000 mL colloid, and 4 units of packed red blood cells (PRBCs). The second procedure lasted 4 hours, EBL was 400 mL, and the patient received 2,700 mL crystalloid, no colloid, and 2 units of PRBCs. Mean arterial pressure (MAP) remained between 55 and 90 mm Hg throughout both surgeries. Hematocrit was 41.1%, 31.9%, and 27.2% before the first surgery, after the first surgery, and after the second surgery, respectively. The corresponding hemoglobin levels were 13.3 g/dL, 10.6 g/dL, and 9.1 g/dL, respectively. The patient had a history of bilateral hip replacements for avascular necrosis 4 years ago with multiple revisions and debridements of the left hip since that time. Two years previously, the patient experienced acute painless vision loss School of Medicine (BKM), Washington University in St Louis, St Louis, Missouri; and Department of Ophthalmology and Visual Sci-ences (MG, IP, GPVS, CMM), Washington University School of Medicine in St Louis, St Louis, Missouri. The authors report no conflicts of interest. Address correspondence to Collin M. McClelland, MD, Department of Ophthalmology and Visual Sciences, Washington University School of Medicine in St Louis, Campus Box 8096, 660 S. Euclid Avenue Street, St Louis, MO 63110; E-mail: mcclellandc@vision. wustl.edu Marshall et al: J Neuro-Ophthalmol 2014; 34: 165-168 165 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. in the left eye after a left hip reimplantation. At that time, the patient reported a left inferior visual field defect 1 week after surgery, but there was no documentation of an ophthalmic examination. At that time, surgery was reported to be uncomplicated, lasting 3.5 hours, EBL of 500 mL, and administration of 5,000 mL of crystalloid. MAP remained between 60 and 100 mm Hg. HIV was detected in the patient 17 years ago and was well controlled on highly active antiretroviral therapy (emtricitabine, tenofovir, and efavirenz) with a recent CD4 count of 529/mL and an undetectable viral load. He had been on the same antiviral regimen for at least 2.5 years. One month after his most recent perioperative vision loss, visual acuity in the right eye was light perception. The right optic disc edema had resolved with development of optic disc pallor. The left eye examination remained unchanged, and left visual field testing showed an inferior altitudinal defect (Fig. 2). DISCUSSION Perioperative vision loss has been defined as occurring within 1 week of a surgical procedure (1,5). The differential diagnosis for perioperative vision loss, along with distinguishing clinical FIG. 1. The right optic disc (A) is diffusely swollen, whereas the left disc (B) has superotemporal pallor. FIG. 2. Kinetic perimetry demonstrates an inferior visual field defect in the left eye. 166 Marshall et al: J Neuro-Ophthalmol 2014; 34: 165-168 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. features of each diagnosis, is shown in Table 1. Ischemic optic neuropathy, either anterior or posterior, is the most common cause of permanent perioperative vision loss. Our patient's history of acute, painless perioperative vision loss in the left eye followed by superior segment optic pallor and an inferior altitudinal visual field defect in a "crowded" optic nerve is highly suggestive of previous perioperative AION. However, without documentation of the optic nerve appearance imme-diately after vision loss, posterior ischemic optic neuropathy (PION) remains a possibility. His most recent episode of vision loss is classic for perioperative AION: sudden painless decline in vision with a RAPD and optic disc edema within days of a surgical procedure. Our patient was not on any medications that may have predisposed him to ION. Although anti-HIV nucleoside analogs such as emtricitabine have been implicated as potential triggers for vision loss in Leber hereditary optic neuropathy (LHON) by inhibiting mitochondrial enzymes (6,7), our patient had no family history of eye disease and the clinical presentation was not suggestive of LHON. Estimates of perioperative ION range from 0.0004% for noncardiac surgeries (8) to 0.12% for spinal surgeries (9), with one study reporting an incidence of 0.013% for all nonocular surgeries performed at a single institution (1). Orthopedic surgeries tend to have a relatively low incidence of ION. Shen et al (10) reported a prevalence of 0.004% after hip surgery. Perioperative AION is most commonly associated with cardiac surgery, although cases have been reported after prostatectomy, liposuction, major vascular surgery (2-4), as well as after hip surgery and other orthopedic procedures (11,12). PION oc-curs most commonly after spinal surgeries, but also has been reported after radical neck dissections and cardiac surgery (2). The degree of vision loss in both AION and PION can range from mild visual field defects to no light perception (13,14). In one study of postoperative vision loss after spine surgery, 42% of patients with AION and 61% of patients with PION had no light perception (15). Although PION commonly presents with vision loss immediately after surgery, patients who develop AION often report normal vision for several days followed by rapid loss (13). Various risk factors may contribute to perioperative ION and can be categorized as intraoperative hemodynamic, intraocular anatomic, and vasculopathic. Intraoperative risk factors include hypotension and anemia resulting from blood loss and volume replacement (14,16-18). Lee et al (15) found that 94% of ION cases after spine surgery had an anesthetic duration of 6 hours or more and 82% of cases had an EBL of 1 liter or more (15). Other proposed intraoperative risk fac-tors include venous congestion, which often occurs during radical neck dissection and spine surgery performed in the Trendelenburg position (19). Increased intraocular pressure also may contribute to the development of AION and is TABLE 1. Differential diagnosis of perioperative vision loss Diagnosis Clinical Presentation Evaluation Central retinal artery occlusion Sudden painless vision loss Pupil examination Possibly signs of external ocular compression RAPD if unilateral or asymmetric Funduscopic examination Retinal edema Cherry red macular spot Attenuated retinal vessels Anterior ischemic optic neuropathy Sudden painless vision loss Pupil examination RAPD if unilateral or asymmetric Funduscopic examination Disc edema with or without peripapillary hemorrhages Posterior ischemic optic neuropathy Sudden painless vision loss Pupil examination RAPD if unilateral or asymmetric Normal funduscopic examination Pituitary apoplexy Visual field defects Neuroimaging Bitemporal hemianopia Junctional scotoma Homonymous hemianopia Ophthalmoplegia Endocrine dysfunction Headache/vomiting Cortical blindness Homonymous visual field defects Normal ophthalmic examination Other neurological deficits No RAPD Neuroimaging RAPD, relative afferent pupillary defect. Marshall et al: J Neuro-Ophthalmol 2014; 34: 165-168 167 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. commonly associated with spine surgery in the prone position (20). Finally, patient-specific vasculopathic risk factors that compromise optic nerve perfusion may be causative and include diabetes mellitus, obesity, and hypertension (16,17,21,22). A small cup-to-disc ratio (disc at risk) may also be a patient-specific risk factor (23,24). Currently, there are no methods proven effective at preventing perioperative ION. Given that intraoperative hypotension and anemia are reported risk factors, numerous preventative techniques have been suggested to address these issues. Potential strategies include careful monitoring and regulating blood pressure, maintaining hematocrit above 30%, minimizing larger crystalloid infusion, posi-tioning the head above the heart, and dividing prolonged surgeries into shorter staged procedures (13). There is no recommended transfusion threshold to eliminate the risk of vision loss (25). Although monitoring of intraoperative optic nerve function has been proposed as a means of detecting optic nerve ischemia, there is no reliable technique to perform this monitoring because the use of visual evoked potentials has been shown to be unreliable in anesthetized patients (26-28). Current recommendations from the American Society of Anesthesiologists Task Force on Peri-operative Blindness incorporate many of the hemodynamic preventive strategies described above and suggest that patients undergoing high-risk procedures be informed of a small chance of vision loss during surgery (25). Our case highlights the difficulty in predicting who will develop ION and also illustrates the importance of patient-specific risk factors for perioperative ION. Surgeons, anesthesiologists, ophthalmologists, and patients should be cognizant that perioperative ION after one surgery may increase the risk of ION after subsequent surgeries. REFERENCES 1. Holy SE, Tsai JH, McAllister RK, Smith KH. Perioperative ischemic optic neuropathy: a case control analysis of 126,666 surgical procedures at a single institution. Anesthesiology. 2009;110:246-253. 2. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531- 546. 3. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145:604-610.e1. 4. Roth S. Perioperative visual loss: what do we know, what can we do? Br J Anaesth. 2009;103:i31-i40. 5. Tidow-Kebritchi S, Jay WM. Anterior ischemic optic neuropathy following off-pump cardiac bypass surgery. Semin Ophthalmol. 2003;18:166-168. 6. Mackey DA, Fingert JH, Luzhansky JZ, McCluskey PJ, Howell N, Hall AJH, Pierce AB, Hoy JF. Leber's hereditary optic neuropathy triggered by antiretroviral therapy for human immunodeficiency virus. Eye (Lond). 2003;17:312-317. 7. Luzhansky JZ, Pierce AB, Hoy JF, Hall AJH. Leber's hereditary optic neuropathy in the setting of nucleoside analogue toxicity. AIDS. 2001;15:1588-1589. 8. Warner ME, Warner MA, Garrity JA, MacKenzie RA, Warner DO. The frequency of perioperative vision loss. Anesth Analg. 2001;93:1417-1421. 9. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22:1319-1324. 10. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109:1534-1545. 11. Kaeser P-F, Borruat F-X. Visual loss after orthopedic procedures. J Arthroplasty. 2011;26:338.e17-338.e19. 12. Bonardo P, Ebner R, Martínez O, Fernández Sasso E, Martínez H, Fernández Pardal M, Reisin R. Bilateral and simultaneous ischemic optic neuropathy following hip replacement surgery. Neurologia. 2000;15:258-260. 13. Lee LA, Newman NJ, Wagner TA, Dettori JR, Dettori NJ. Postoperative ischemic optic neuropathy. Spine (Phila Pa 1976). 2010;35:S105-S116. 14. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50:15-26. 15. Lee LA, Roth S, Posner KL, Cheney FW, Caplan RA, Newman NJ, Domino KB. The American Society of Anesthesiologists postoperative visual loss registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105:652-659; quiz 867-868. 16. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33:1491-1496. 17. Nuttall GA, Garrity JA, Dearani JA, Abel MD, Schroeder DR, Mullany CJ. Risk factors for ischemic optic neuropathy after cardiopulmonary bypass: a matched case/control study. Anesth Analg. 2001;93:1410-1416, Table of contents. 18. Shapira OM, Kimmel WA, Lindsey PS, Shahian DM. Anterior ischemic optic neuropathy after open heart operations. Ann Thorac Surg. 1996;61:660-666. 19. Marks SC, Jaques DA, Hirata RM, Saunders JR Jr. Blindness following bilateral radical neck dissection. Head Neck. 1990;12:342-345. 20. Cheng MA, Todorov A, Tempelhoff R, McHugh T, Crowder CM, Lauryssen C. The effect of prone positioning on intraocular pressure in anesthetized patients. Anesthesiology. 2001;95:1351-1355. 21. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology. 2012;116:15-24. 22. Jangra K, Grover V. Perioperative vision loss: a complication to watch out. J Anaesthesiol Clin Pharmacol. 2012;28: 11-16. 23. Beck RW, Savino PJ, Repka MX, Schatz NJ, Sergott RC. Optic disc structure in anterior ischemic optic neuropathy. Ophthalmology. 1984;91:1334-1337. 24. Beck RW, Servais GE, Hayreh SS. Anterior ischemic optic neuropathy. IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987;94:1503-1508. 25. Practice advisory for perioperative visual loss associated with spine surgery: a report by the American Society of Anesthesiologists Task Force on Perioperative Blindness. Anesthesiology. 2006;104:1319-1328. 26. Wiedemayer H, Fauser B, Sandalcioglu IE, Armbruster W, Stolke D. Observations on intraoperative monitoring of visual pathways using steady-state visual evoked potentials. Eur J Anaesthesiol. 2004;21:429-433. 27. Cedzich C, Schramm J, Fahlbusch R. Are flash-evoked visual potentials useful for intraoperative monitoring of visual pathway function? Neurosurgery. 1987;21:709-715. 28. Raudzens PA. Intraoperative monitoring of evoked potentials. Ann N Y Acad Sci. 1982;388:308-326. 168 Marshall et al: J Neuro-Ophthalmol 2014; 34: 165-168 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |