| OCR Text |
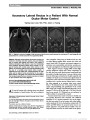
Show Positional Suppression of Periodic Alternating Nystagmus Seok Jong Chung, MD, Seong-Ho Park, MD, PhD, Ji-Soo Kim, MD, PhD, Sea Won Oh, BSc, Hyo Jung Kim, MSc, Jae Han Park, MD, Young Eun Huh, MD Abstract: A 43-year-old man with a high-grade glioma involving the cerebellar nodulus showed a near-complete suppression of periodic alternating nystagmus (PAN) in the lateral decubitus position to either side. This positional modulation of PAN is consistent with suppression of the velocity storage mechanism by head position changes (tilt dumping) and is supportive of the role of the velocity storage mechanism in generating PAN. Journal of Neuro-Ophthalmology 2014;34:162-164 doi: 10.1097/WNO.0000000000000098 © 2014 by North American Neuro-Ophthalmology Society Periodic alternating nystagmus (PAN) is characterized by a horizontal jerk nystagmus, which reverses its direction periodically with a brief transition period (1). Although the pathogenesis of PAN remains uncertain, it has been ascribed to the instability of the velocity storage mechanism because of lesions involving the cerebellar nodulus or its connections with the vestibular nuclei and related structures (2). Because the velocity storage mechanism is suppressed by head posi-tion changes in the direction of gravity (tilt dumping) (3), positional changes may affect PAN by altering the gravicep-tive inputs to the nodulus. CASE REPORT A 43-year-old man was referred for evaluation of dizziness and imbalance for 3 months. Examination showed sponta-neous horizontal nystagmus that changed directions approx-imately every 2 minutes with a brief transition period for several seconds. Downbeat nystagmus became evident during the transition period (See Supplemental Digital Content, Video 1, http://links.lww.com/WNO/A93). While looking straight ahead in the sitting position, 1 cycle of PAN con-sisted of alternating left- and right-beating nystagmus with each half-cycle lasting approximately 110 seconds and an intervening transition period of approximately 15 seconds (Fig. 1A; See Supplemental Digital Content, Video 1, http://links.lww.com/WNO/A93). During each half-cycle, the nystagmus gradually built up and then decreased with a maximum slow phase velocity reaching approximately 7°/s in either direction (Fig. 1A). Horizontal head shaking, head impulses, and vibratory stimulation on the brow or the mas-toids did not affect the intensity or periodicity of the PAN. The patient underwent various positional changes includ-ing head tilt to either side, head bending while sitting, lying on his back, lying on his face, and lying on either side. The PAN dissipated over about 2 cycles while lying on either side, regardless of the initial direction of nystag-mus (Fig. 1B; See Supplemental Digital Content, Video 2, http://links.lww.com/WNO/A94). In contrast, the basic pattern of PAN was maintained in other positions although the periodicity or the intensity of PAN was slightly affected (Fig. 1A). Head impulse vestibulo-ocular reflex, bithermal caloric tests, fundus photography, ocular vestibular evoked myogenic potentials (VEMP), and pure tone audiogram were normal. Department of Neurology (SJC), Yonsei University College of Medi-cine, Seoul, Korea; Department of Neurology (S-HP, J-SK, SWO, HJK, JHP, YEH), Seoul National University Bundang Hospital, Seongnam-si, Korea; and Department of Neurology (S-HP, J-SK), Seoul National University College of Medicine, Seoul, Korea. S. J. Chung, MD and S-H. Park equally contributed to this study. S. J. Chung, S-H. Park, S. W. Oh, H. J. Kim, J. H. Park, and Y. E. Huh report no disclosures. J. S. Kim serves as an Associate Editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Neuro-ophthalmology, Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, and Journal of Vestibular Research; and received research support from SK Chemicals, Co. Ltd. Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www. jneuro-ophthalmology.com). Supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A080750). Address correspondence to Ji-Soo Kim, MD, PhD, Department of Neurology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 300 Gumi-dong, Bundang-gu, Seongnam-si, Gyeonggi-do 463-707, Korea; E-mail: jisookim@snu.ac.kr 162 Chung et al: J Neuro-Ophthalmol 2014; 34: 162-164 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Brain magnetic resonance imaging (MRI) revealed enhancement around the anteroinferior cerebellum includ-ing the nodulus and flocculus (Fig. 1C), in the right medial temporal and suprasellar areas, and perimesence-phalic cistern and internal auditory canals. Brain positron emission tomography (PET) showed hypermetabolism in the corresponding regions (Fig. 1D). The patient under-went right temporal lobectomy, and the pathology was consistent with a high-grade glioma. DISCUSSION PAN has been ascribed to nodular lesions and resulting disinhibition of the velocity storage mechanism that normally enhances the vestibular responses during lower-frequency stimulation (4,5). The nodulus has connections with the vestibular nuclei that take part in the velocity storage mech-anism (6,7). Thus, spontaneous nystagmus augmented by increased vestibular storage activities would reverse the direc-tion periodically because of a normal vestibular repair Fig. 1. Recording of periodic alternating nystagmus, magnetic resonance imaging, and positron emission tomography (PET). A. While looking straight ahead in the sitting position, 1 cycle of periodic alternating nystagmus (PAN) consists of alternating left- and right-beating nystagmus with each half-cycle lasting for approximately 110 seconds and an intervening transition period of approximately 15 seconds. During each half-cycle, the nystagmus gradually builds up and then decreases with a maximum slow phase velocity reaching approximately 7°/s in either direction. While lying down, the right-beating nys-tagmus tends to increase slightly while the left-beating nystagmus decreases without a change in the periodicity. B. The PAN dissipates over about 2 cycles while lying on either side. C. Postcontrast T1 axial magnetic resonance images show enhancement along the nodulus and flocculus. D. PET reveals hypermetabolism in the corresponding regions after intra-venous 18F-fluorodeoxyglucose. Chung et al: J Neuro-Ophthalmol 2014; 34: 162-164 163 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. mechanism (8). In our patient, the enhancing MRI lesions and PET hypermetabolism in the area of the nodulus also support the nodulus as the neural substrate of PAN. The nodulus is known to play an important role in the processing of otolithic signals through its connection with the otolithic organs, and it gives rise to positional nystagmus when damaged (8). Previously, apogeotropic positional nys-tagmus was induced during head turning in patients with nodular lesions while in the supine position (9). Accord-ingly, the role of apogeotropic positional nystagmus may be considered in the positional modulation of PAN in our patient. However, the suppression of both half-cycles of PAN and eventual resolution of the nystagmus during side-lying to either side do not support the role of apogeo-tropic positional nystagmus. Otherwise, the otolithic signal generated during the side-lying may have modulated the activity of the velocity storage mechanism. Indeed, the time constant of the postrotatory nystagmus is shortened when the head is tilted or bent down at the onset of postrotatory nystagmus, and this tilt suppression (dumping) is diminished in nodular lesions (3). The nodular dysfunction should have been partial in our patient to explain the positional modula-tion of PAN. It is unknown why the positional effect was greatest when the patient assumed a side-lying position. Because the direction of the PAN was horizontal, the possible effect of the gravity on PAN would have been maximal when it was mostly aligned with the interaural axis. The positional modulation of PAN in our patient differs from the findings of Furman et al (2) who showed no significant modulation with changes in static head position in 4 patients with PAN. The reason for this discrepancy is unclear. The completeness of nodular dysfunction or contribution of other cerebellar structures may be considered because the patients in the previous study (2) had diffuse cerebellar pathology. Another explanation may be peripheral vestibular involvement from leptomeningeal seeding around the brainstem and internal auditory canals as demonstrated on MRI. This may have altered the otolithic signals although this seems unlikely given the normal results of head impulse and caloric testing, ocular VEMP, and audiometry. In conclusion, the positional modulation of PAN in our patient is consistent with suppression of the velocity storage mechanism by head position changes and is supportive of the role of the velocity storage mechanism in generating PAN. REFERENCES 1. Rudge P, Leech J. Analysis of a case of periodic alternating nystagmus. J Neurol Neurosurg Psychiatry. 1976;39:314-319. 2. Furman JM, Wall C III, Pang DL. Vestibular function in periodic alternating nystagmus. Brain. 1990;113:1425-1439. 3. Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228:199-202. 4. Oh YM, Choi KD, Oh SY, Kim JS. Periodic alternating nystagmus with circumscribed nodular lesion. Neurology. 2006;67:399. 5. Jeong HS, Oh JY, Kim JS, Kim J, Lee AY, Oh SY. Periodic alternating nystagmus in isolated nodular infarction. Neurology. 2007;68:956-957. 6. Voogd J, Gerrits NM, Ruigrok TJ. Organization of the vestibulocerebellum. Ann N Y Acad Sci. 1996;781:553-579. 7. Solomon D, Cohen B. Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res. 1994;102:57-68. 8. Sheliga BM, Yakushin SB, Silvers A, Raphan T, Cohen B. Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula of the vestibulocerebellum. Ann N Y Acad Sci. 1999;871:94-122. 9. Nam J, Kim S, Huh Y, Kim JS. Ageotropic central positional nystagmus in nodular infarction. Neurology. 2009;73:1163. 164 Chung et al: J Neuro-Ophthalmol 2014; 34: 162-164 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |