| OCR Text |
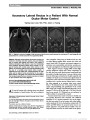
Show The Idiopathic Intracranial Hypertension Treatment Trial: Design Considerations and Methods Deborah I. Friedman, MD, MPH, Michael P. McDermott, PhD, Karl Kieburtz, MD, MPH, Mark Kupersmith, MD, Ann Stoutenburg, CCRC, John L. Keltner, MD, Steven E. Feldon, MD, MBA, Eleanor Schron, PhD, RN, James J. Corbett, MD, Michael Wall, MD; for the NORDIC IIHTT Study Group Background: The objectives of this study were to present the rationale for the main aspects of the study design and describe the trial methodology for the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT). Methods: Eligible candidates with mild visual field loss (automated perimetric mean deviation [PMD] 22 to 27 dB) were randomized to receive either acetazolamide or matching placebo tablets. Randomized participants were offered partic-ipation in a supervised dietary program. The primary outcome variable, PMD, was measured at 6 months. Additionally, cere-brospinal fluid from subjects and serum from study partici-pants and matched controls were collected for genetic analysis and vitamin A studies. An ancillary optical coherence substudy was added to investigate the changes of papillede-ma in the optic nerve head and retina that correlate with Frisén grading, visual field deficits, and low-contrast visual acuity. Results: The randomized trial entered 165 participants from March 17, 2010, through November 27, 2012, from the United States and Canada. The primary outcome (month 6) visits were successfully completed by June 15, 2013. Blood specimens were obtained from 165 controls without IIH to investigate vitamin A metabolism and genetic markers of potential risk factors for IIH. Conclusions: The IIHTT is the first randomized, double-masked placebo-controlled trial to study the effectiveness of medical treatment for patients with IIH. Journal of Neuro-Ophthalmology 2014;34:107-117 doi: 10.1097/WNO.0000000000000114 © 2014 by North American Neuro-Ophthalmology Society Idiopathic intracranial hypertension (IIH), also called pseu-dotumor cerebri, is a disorder of elevated intracranial pres-sure of unknown cause (1). Its yearly incidence is 22.5 per 100,000 in overweight women of childbearing age and is rising in parallel with the obesity epidemic (2,3). It affects about 100,000 Americans. Most patients suffer debilitating headaches. Because of pressure on the optic nerve (papille-dema), 86% have some degree of permanent visual loss and 10% develop severe visual loss (4). Although a number of interventions have been used in clinical practice to prevent loss of sight, none has been proven effective. The most recent Cochrane review stated, "There is insufficient information to generate an evidence-based management strategy for idio-pathic intracranial hypertension. Of the various treatments available, there is inadequate information regarding which are truly beneficial and which are potentially harmful. Properly designed and executed trials are needed" (5). No randomized clinical trial in patients with visual loss from IIH had been performed before the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) for several reasons. IIH is officially classified as a "rare disease" by the National Institutes of Health (www.rarediseases.info. nih.gov), raising concern over the feasibility of recruiting a sufficiently large sample of participants. A wide variety Departments of Neurology & Neurotherapeutics and Ophthalmol-ogy (DIF), University of Texas Southwestern Medical Center, Dallas, Texas; Departments of Ophthalmology, Neurology, Neurosurgery and Visual Science (SEF), Center for Human Experimental Thera-peutics (KK, AS), Department of Biostatistics and Computational Biology and Department of Neurology (MM), University of Rochester School of Medicine and Dentistry, Rochester, New York; Neurology (MW), University of Iowa College of Medicine and Iowa City Veterans Affairs Health Care System, Iowa City, Iowa; National Eye Institute (ES), Bethesda, Maryland; Department of Ophthal-mology and Vision Science (JK), University of California Davis Medical Center, Sacramento, California; Departments of Neurology and Ophthalmology (MK), Mount Sinai School of Medicine, New York, New York. Supported by National Eye Institute (1U10EY017281-01A1, 1U10EY017387-01A1, 3U10EY017281-01A1S1, 3U10EY017281-01A1S2). The authors report no conflicts of interest. Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www. jneuro-ophthalmology.com). Address correspondence to Deborah I. Friedman, MD, MPH, Uni-versity of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, MC 9036, Dallas, TX 75390-9036; E-mail: Deborah.Friedman@ utsouthwestern.edu Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 107 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. of treatments are used in clinical practice, including dietary interventions, carbonic anhydrase inhibitors, diuretics, cere-brospinal fluid diversion procedures (shunts), and optic nerve sheath fenestration (ONSF). The indications for var-ious treatments are not well defined, and there has been considerable controversy regarding the threshold for using surgical therapy, as well as which surgical therapy is prefer-able to study. No prospective studies have shown a specific therapy to control or improve IIH symptoms and signs or to consistently prevent or reverse vision deterioration. Nonetheless, many neuro-ophthalmologists were uncom-fortable incorporating a placebo group in a treatment trial of IIH, believing that some type of active treatment was necessary despite the lack of evidence. Thus, although ex-perts in the field convened annually to discuss the possibil-ity of a trial for almost 20 years, they were unable to concur on the intervention to be studied, and the subpopulation of patients to include, the nature of the control group, and the outcome variables to be assessed. With the epidemic increase in obesity in the United States, Canada, and many countries outside of North America, the incidence of IIH is expected to continue rising (4). The morbidity and health issues associated with IIH have a socioeconomic impact on the affected individual as well as the health care system. Because of resulting disability from debilitating headaches and vision loss and the limited scien-tific rationale to guide treatment decisions, the National Eye Institute determined that critically evaluating the cause, course, and treatment of IIH was an institutional priority. Their charge was to establish convincing evidence-based treat-ment strategies for IIH to prevent unnecessary visual loss. When the study steering committee for the IIHTT began to consider study design, they had many issues to resolve such as selecting the study intervention (medical therapy, surgery, or both), determining the time of onset of illness for eligibility purposes, determining the appro-priate management strategy for the control group, select-ing the visual status at the time of eligibility to study (i.e., mild, moderate, or severe visual loss), inclusion of adolescents, and, if drug therapy were chosen, the study drug and dosing strategy. Once the basic study design was agreed upon, other considerations included methods to ensure effective placebo masking of acetazolamide given the known side effects of the drug, selecting a cost-effective and uniform dietary intervention to be used across multiple centers, accounting for a possible thera-peutic effect of the diagnostic lumbar puncture (LP), procedural integrity and uniformity of the diagnostic LP across sites, type of neuroimaging study required before study entry, which visual measure (i.e., acuity, visual field, papilledema grade) best represents a clinically meaningful primary outcome, documentation and quantitation of papilledema, potentially causative risk factors to study, and appropriate quality of life indices to measure in this population. Surveys, pilot data, and reviews of the existing literature were used to gain further clarity regarding issues affecting study design and to assess the feasibility of performing the trial. Ultimately, the IIHTT included a randomized, double-masked placebo-controlled treatment trial, a nested case-control study to investigate possible etiologies of the disease, and an ancillary study of optical coherence tomog-raphy (OCT) in IIH. RANDOMIZED TREATMENT TRIAL Study Objectives The IIHTT was a multicenter, randomized, double-masked placebo-controlled trial to determine the efficacy of acetazol-amide (up to 4 g/d) compared with placebo, with everyone receiving intervention with a supervised weight-reduction program, in reducing or reversing visual loss after 6 months of intervention (Fig. 1). The study population were individ-uals with IIH and mild visual field loss (initially baseline perimetric mean deviation [PMD] of 22 to 25 dB, later expanded to include PMD up to 27 dB, in the most affected eye). The primary outcome variable of PMD in the most affected eye (study eye) at randomization was analyzed at 6 months. Randomized participants were followed up to 12 months and yearly thereafter. Additional objectives included determining associations between the study inter-ventions and visual field defects, Frisén papilledema grade, and the severity and frequency of headaches. Study Population Preliminary Data and Rationale for Recruitment Potential In anticipation of potentially needing 200 or more participants for a treatment trial, an initial survey was done by the IIH Study Group in 2000 to determine the numbers of patients with IIH seen in 18 Neuro-ophthalmology FIG. 1. Study design of idiopathic intracranial hypertension treatment trial (IIHTT). 108 Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. centers. Patients aged 18-60 years meeting the modified Dandy criteria (6) for IIH were included in the survey. De-identified data were collected from 380 patients (35 men and 345 women) seen in a 1-year period from June 1999 through June 2000. Treatments used included acet-azolamide, furosemide, or other diuretics (71%), a weight reduction diet alone (19%), lumboperitoneal shunt (12%), ONSF (12%), corticosteroids (3%), and repeated LP (1%), and 4% received no treatment. Some patients were treated with multiple interventions. These data documented that IIH is encountered frequently enough in a Neuro-ophthalmology practice to support adequate recruitment, that management regimens vary considerably and that acet-azolamide and other diuretics were the most frequently used treatments. IIH Randomized Participants The inclusion and exclusion criteria are listed in Table 1. Adolescents were not included because previous experience by the New York Obesity Nutrition Research Center (NYONRC) found that children in this age group had dif-ficulty adhering to a dietary protocol, which was a required co-intervention in this study. Use of topiramate, corticoste-roids, and other diuretics was not permitted during the double-masked phase of the trial. Human Subjects Protection and Regulatory Approvals The IIHTT protocol was approved by the Research Subjects Review Board at the University of Rochester and the Institutional Review Boards (IRBs) at the University of Iowa and St. Luke's Roosevelt Hospital before being sent out to the study sites. The study was approved by IRBs at all sites, and at sites without a governing IRB, by Western IRB. All treatment trial participants and control subjects pro-vided written informed consent. The IIHTT was registered on clinicaltrials.gov. As acetazolamide is not approved by the Food and Drug Administration (FDA) for the treatment of IIH, an investigational new drug (IND) application was submitted. The FDA and Health Canada waived the need for an IND for the IIHTT. Outcomes for Treatment Intervention Primary Outcome Variable: Perimetric Mean Deviation Rationale We used PMD on the Automated Visual Field Analyzer as a global measure of visual function and as an outcome. We chose PMD because of its more stable retest variability compared with individual or groups of test locations and its sensitivity to global clinically meaningful changes in IIH (7). Papilledema grade was considered but not used as a pri-mary outcome variable because "improvement" may also occur as optic atrophy develops. To further define the range of PMD acceptable for inclusion, the IIHTT Steering Committee members performed a retrospective chart review of 154 consecutive patients who met the modified Dandy criteria for IIH from 5 Neuro-ophthalmology centers. All patients had follow-up visits within 4-12 months with manual or automated perimetry. Data were abstracted from 109 eligible patient charts. The presenting PMD ranged from 235 to +1.29 dB with a median of 23.11 dB. Of the 109 patients, 14 had no visual loss and 48 had mild visual loss (PMD: 22 to 25 dB). Thirty-seven patients had a PMD of 22 dB or worse but better than 25 dB. There were 26 patients with moderate visual loss, defined as PMD 25 dB to 214 dB, and 13 patients had a PMD worse than 214 dB. In patients with mild visual loss, the mean ± standard deviation (SD) change in PMD in the worst affected eye over 6.4 ± 1.9 months of observation was 0.82 ± 2.35 dB. These data were used to plan the sample size for the IIHTT. The initial visual metric for study eligibility was a PMD from 22 to 25 dB as most neuro-ophthalmologists treat this range of visual loss medically in practice. Patients with a PMD better than 22 dB at baseline were excluded because a normal PMD precluded the ability to assess significant improvement over time. During the course of the study, the visual field eligibility metric was expanded to include cases with a PMD up to 27 dB to facilitate recruitment. IIHTT Visual Field Procedure Automated perimetry was performed using the SITA Stan-dard program 24-2 in both eyes. The testing was performed by a technician certified by the Visual Field Reading Center (VFRC) using the IIHTT manual of procedures for the VFRC. Each candidate had at least 2 initial visual field examinations done at least 1 hour apart. As the primary outcome variable was change in PMD from baseline to month 6 in the eye with the worse PMD at baseline, potential enrollees were required to provide reliable and reproducible visual field examinations (i.e., able to maintain fixation using an eye monitoring device, ,15% false-positive errors) at the screening visit to be eligible for participation. Because the LP can transiently lower intracranial pressure and potentially improve visual function, the study used the following protocol: the baseline PMD was the average of the PMD from 2 qualifying visual fields. If the patient had an LP done more than 1 week before the screening visit, qualifying perimetric testing was performed during the first visit. If the LP had not yet been performed, a single perimetric test was done on each eye before the LP. The second test for each eye was done after the LP. If the VFRC determined that the initial 2 visual field results agreed and met VFRC reliability standards, regardless of the timing of the LP, these visual fields served as the qualifying baseline visual fields. If they did not agree, a third visual field was performed on the study eye. If the visual field was reliable, the third field served as one of the 2 qualifying baseline fields, but if it was unreliable, another visual field was performed. Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 109 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. The vision in the eye with the most perimetric visual loss based on PMD was considered the study eye for the purposes of the primary outcome assessment, but both eyes were tested and followed. The results of the fellow eye testing were used as a safety measure (treatment failure could occur in the less affected eye) and as a secondary outcome variable. Both eyes were evaluated for progression after each visit as part of data and safety monitoring. Secondary Outcomes: Quality of Life Assessment: Rationale Quality of Life (QOL) instruments were incorporated into the IIHTT based on studies showing a substantial impact of IIH in multiple domains, including depression, physical functioning, role functioning, bodily pain and general health compared with weight-matched controls (8), neuro-ophthalmologic controls, and disease-free individuals TABLE 1. Inclusion and exclusion criteria for IIHTT Inclusion Criteria 1. Age 18-60 years at the time of diagnosis 2. Diagnosis of IIH by modified Dandy criteria (6) a. LP opening pressure .250 mm CSF with normal CSF contents If opening pressure 200-250 mm CSF, at least one of the following: i. Pulse-synchronous tinnitus ii. Abducens nerve palsy iii. Grade II papilledema iv. No evidence of pseudopapilledema v. Lateral sinus stenosis or collapse on MRV vi. Partially empty sella with unfolded perioptic nerve CSF spaces b. Bilateral papilledema 3. Reproducible visual field loss on automated perimetry with an average perimetric mean deviation 22 to 27 dB in the worst eye 4. Able to provide informed consent 5. Agree to use an acceptable form of birth control during the intervention phase of the study if of childbearing potential Exclusion criteria 1. Treatment for IIH for more than 2 weeks with any agent (acetazolamide was permitted for 1 week; 1 day of washout was required for every day of acetazolamide treatment before the screening visit) 2. Previous surgery, endovascular procedures, or bariatric surgery for IIH treatment 3. Abnormalities on neurologic examination aside from papilledema and its related visual loss or abducens nerve paresis 4. Abnormal CT or MRI (e.g., intracranial mass, hydrocephalus, dural venous sinus thrombus, or arteriovenous malformation) except for an empty sella, unfolded optic nerve sheaths, flattened sclera, or smooth-walled venous sinus stenosis 5. Opening pressure ,200 mm CSF (a repeat CSF pressure measurement was allowed if the first LP was normal, improperly performed, or no opening pressure was obtained) 6. Exposure to an oral drug, substance, or disorder that has been associated with elevation of intracranial pressure within 2 months of diagnosis (lithium, vitamin A, tetracycline and related compounds) 7. Diagnosed untreated obstructive sleep apnea 8. Condition requiring diuretics, oral, intravenous or injectable steroids, or other potential pressure-lowering agents including topiramate (nasal, inhaled, or topical steroids were permitted) 9. Intraocular pressures currently .28 or .30 mm Hg at any time in the past; Refractive error greater than ±8.00 sphere or more than ±3.00 cylinder in either eye was ineligible if there were abnormalities on ophthalmoscopy or fundus photos related to myopia that are associated with visual loss (e.g., staphyloma, retinal thinning in the posterior pole, or more than mild optic disc tilt) 10. Hyperopia greater than +6.00 D but less than or equal to +8.00 D sphere if there was no characteristic halo of peripapillary edema as determined by the site investigator or the PRC 11. Other disorders causing visual loss except for refractive error and amblyopia including vitreous cells iritis, or optic disc drusen on examination or on previous encounters 12. A known allergy to pupil dilating drops or narrow angles precluding safe dilation 13. Abnormal blood work or a medical condition associated with raised ICP or a contraindication to taking acetazolamide (severe anemia, leukopenia or thrombocytopenia, renal failure, hepatic disease, renal stones) 14. Type 1 diabetes or the presence of diabetic retinopathy 15. Pregnancy or unwillingness for subject of childbearing potential to use contraception during the first year of the study 16. Breastfeeding mothers (unless willing to discontinue breastfeeding by the baseline visit) CSF, cerebrospinal fluid; CT, computed tomography; ICP, intracranial pressure; IIH, idiopathic intracranial hypertension; IIHTT, Idiopathic Intra-cranial Hypertension Treatment Trial; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; PRC, photography reading center. 110 Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. (9). Vision-related QOL, as assessed by the 25-Item National Eye Institute Visual Functioning Questionnaire (VFQ-25) also have been found to be significantly lower in individuals with IIH compared with neuro-ophthalmologic and disease-free controls (9). In a separate pilot study, 10 women with IIH completed 2 headache questionnaires and participated in semistructured telephone interviews and focus groups to assess the content domains of the Beck Depression Inventory, VFQ-25, Patient Health Questionnaire, Migraine Disability Assessment, and Headache Impact Test (HIT-6) scales for use in future research of IIH (10). Based on their responses, the HIT-6 was selected to measure headache disability in the IIHTT. IIHTT QOL Procedure For vision-related QOL, the NEI VFQ 25, the 10-item Neuro-ophthalmic Supplement to the VFQ-25, and Version 2 of the Medical Outcomes Study (SF-36) were administered at study entry, 6 months, and 12 months. Other assessments included the generic health-related QOL instrument SF-36 (11) and the HIT-6 inventory, a 6-question instrument assessing the impact of headache over the preceding month (12). Participants had the option of completing QOL assessments on the Neuro- Ophthalmology Research Disease Investigator Consortium (NORDIC) Web site in advance of each study follow-up visit. Ophthalmologic Examination The best corrected visual acuity was measured using early treatment diabetic retinopathy study charts and a refraction protocol that was based on the strategy used in the Optic Neuritis Treatment Trial (13). Complete ophthalmologic examinations were performed. Papilledema was graded by the site investigator using the Frisén scale grade (14). Fundus Photography Study photographers were certified by the Photography Reading Center (PRC). Digital fundus photographs cen-tered on the optic disc and the papillomacular area were taken at each visit and sent to the PRC to document the papilledema grade. Cerebrospinal Fluid Pressure Measurement In addition to the baseline LP, all participants were encouraged to have a second LP for cerebrospinal fluid (CSF) pressure measurement at 6 months for comparison. Study Interventions Randomized Intervention After reviewing various options for intervention, the decision was made to study individuals with mild visual loss who were unlikely to require surgical intervention. Although it is generally the first-line medication used for the treatment of IIH in clinical practice worldwide, acetazol-amide had only limited supporting literature (15-18). Case reports suggested dosing up to 4 g daily for IIH treatment (17,18). Polled members of the North American Neuro- Ophthalmology Society generally prescribed between 1- and 2 g daily, and a small number indicated that they would be comfortable using 3-4 g daily in a clinical trial setting. Possible acetazolamide-associated adverse events were considered when designing the trial, particularly with regard to aplastic anemia and hypokalemia. Other diuretic therapy was excluded during the IIHTT based on a study of 92 patients taking acetazolamide who showed no evidence of clinically significant hypokalemia unless they were on other diuretics (19). Although aplastic anemia is idiosyncratic and unpredictable, routine monitoring of the blood count was required by the FDA and serum potassium levels were fol-lowed for safety purposes. Eligible consenting trial participants were randomized with equal probability to acetazolamide (250 mg) or matching placebo tablets. The initial dosage of study drug was 4 tablets daily in 2 divided doses. Higher dosing frequencies were deemed too complicated for the purposes of dose titration and likely to result in decreased adherence. After the initial dosing, the participants scheduled dosage increases of 1 tablet every 6 days up to a maximum dosage of 16 tablets daily (4 g daily for those receiving acetazolamide). Those individuals who were unable to tolerate the study drug could gradually decrease the dosage to a minimum of one-half tablet daily. Starting at the month 6 visit, participants were transitioned off study medication to open-label acetazolamide by substitut-ing 1 acetazolamide 250 mg tablet for 1 tablet of study medication every 6 days to a final dosage of 4 g daily or to a maximally tolerated dosage. This process was incorporated to ensure that subjects who had benefitted from active treatment were maintained on active treatment without revealing the initial treatment assignment. Participants who did not tolerate 1 g of acetazolamide daily could gradually decrease the dosage to a minimum of 125 mg daily. To avoid treating anyone (who may have initially been assigned to placebo) unneces-sarily, any participant with grade 0-1 papilledema was tapered off the study drug but not placed on acetazolamide. However, those with grade 0-1 papilledema who had persisting head-aches, pulse-synchronous tinnitus, or PMD that failed to improve were transitioned to acetazolamide. All participants were off study drug by the month 9 follow-up visit and were seen for a study visit to ensure that their vision was stable after the transition off of study medication. After the 9-month visit, medication was pre-scribed by their treating physician. Unscheduled visits and unscheduled laboratory testing were performed as needed. Intervention Provided to All Participants A specific dietary plan and lifestyle modification program was offered to all treatment trial participants. The rationale was that offering an intervention to all potential enrollees would facilitate recruitment and retention. Regression of Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 111 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. papilledema and symptoms in IIH has been demonstrated with modest weight loss (20-22). The NYONRC developed a telephone-based, uniform weight loss, and lifestyle modification intervention that could be implemented for participants recruited at sites throughout North America (23-25). The intervention covered all 3 disciplines of weight loss and lifestyle modification, that is, nutrition, physical activity, and behavior. All participants were assigned to a weight loss coach for telephone counseling sessions and were given pedometers for measuring steps and rubberized exercise tubing for resistance exercise. Nutritional instruction included meal planning, calorie counting, portion control, fat counting, and energy density. The diet intervention was implemented in 2 phases. During the first 6 months of the study (phase 1), participants were given a 500- to 1000- calorie deficit goal using the Mifflin et al method (26) to determine their calorie needs and instructed on following a partial meal replacement (PMR) diet. Studies show that weight loss is greater for patients using PMR diets compared with reduced-calorie diets; PMR dieters lose 7%-8% body weight compared with 3%-7% for conventional dieters (27). The meal replacements were in the form of shakes, soups, and bars. Weight loss coaches guided participants who declined to use these products to pre-prepared food in the marketplace that is portion and calorie controlled. Counsel-ing sessions with weight loss coaches occurred weekly. A goal of 10% weight decrease from baseline on average was pro-jected during the study. We anticipated that most partici-pants in the study would undergo a weight loss intervention. A low sodium component was included in the event that normal-weight persons entered the trial to ensure that all enrollees had dietary management (28). In months 7-12 (phase 2), participants had the option to transition to a balanced-deficit diet (BDD) or continue with the PMR diet. The BDD was modeled after the Die-tary Approaches to Stop Hypertension (29) and Therapeu-tic Lifestyle Diet of the National Cholesterol Education Program (30). This diet contained approximately 20% of calories from protein, 25%-30% from fat, and the remain-der from carbohydrate. Additional information and instruc-tions were provided on a low-energy-dense diet (31), portion control (32), and environmental influences on diet (33). Counseling sessions occurred biweekly during months 7 and 8, and monthly thereafter. All participants were instructed to keep daily records of their food and beverage intake. A password-protected Internet message board was established for participants to use to communicate with each other as well as with the NYONRC staff. The board also contained links to educational resources. To emphasize the importance of maintaining their weight loss goal, the dietary intervention was offered to participants throughout their participation in the study, even if they were no longer taking study medication. Study Design Randomization Randomization was stratified by site and included blocking to ensure balance among the treatment groups within a site after a certain number of subjects had been enrolled at that site. The order of assignment of the randomization numbers within each site was provided to a masked Data Coordina-tion and Biostatistics Center (DCBC) information analyst so that this information could be incorporated in the web-based enrollment module. A back-up system was in place whereby sites could call the DCBC to randomize a participant manually if necessary. The only individuals with access to the treatment assignments during the trial before database lock were the unmasked programmer in the DCBC who generated the randomization plan, an unmasked statistician in the DCBC who served as a liaison with the independent Data and Safety Monitoring Board (DSMB), and unmasked staff at the Clinical Materials Services Unit (CMSU). These individuals did not communicate with any other staff involved in the trial about study-related matters. Site Personnel and Masking Site personnel included a site principal investigator (PI), treating sub-investigator (TSI), site coordinator, visual field technician(s), and fundus photographer(s). Sites participat-ing in the OCT ancillary study also had an OCT technician. The PI screened study candidates and determined their eligi-bility. All site personnel were masked to the study drug assign-ment. After randomization, all inquiries pertaining to the study drug and adverse events were directed to the site coordinator and Treating Sub-investigation (TSI) to minimize possible bias of the PI to the treatment assignment. The PI was responsible for assessing the subjects for possible treatment failure during the double-masked phase of the study, and for determining whether participants would continue in the open-label phase or taper off study drug without further medication at 6 months. All laboratory data generated after the screening process were reviewed by the TSI. Sample Size Calculation and Data Analysis Plan Sample Size Determination Preliminary data from the IIH Study Group were used to estimate the standard deviation (SD) of the primary outcome variable, 6-month change in PMD. Data on changes in PMD over a period of 6.4 ± 1.9months were available for 37 patients with mild visual loss (22 to 27 dB). In the most affected eye, the mean (±SD) change in PMD was 0.82 ± 2.35 dB. Under the assumption of an SD of 2.35, an initial sample size of 140 subjects (70 per group) was calculated to provide 90% power to detect a group difference in mean change of 1.3 dB, at a significance level of 5% (two-tailed) using a t test. The target sample size was increased to 154 subjects (77 per group) to account for an anticipated withdrawal rate of approximately 10%. Monitoring of the withdrawal rate during the trial 112 Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. revealed this rate to be higher than anticipated and the final sample size was inflated to 165 subjects. The effect size of 1.3 dB was chosen on the basis of a pilot study conducted before the trial as it reflected the minimal difference associated with a clinically significant change in the visual field. Twenty-five charts of patients with IIH with mild visual loss who had at least 5 longitudinal visual field examinations were identified. Each of 3 readers reviewed all visual fields masked to the PMD associated with the field. The criterion for a clinically significant change in the field was a priori defined as a change from the index examination, confirmed on 2 consecutive subsequent examinations, which in the opinion of the reader would require a change in therapy for the patient. Agreement among at least 2 of the 3 participating readers was required for a change to be deemed clinically significant; in fact, all 3 readers were in agreement for 23 of the 25 patients. A clinically significant change was identified in 19 of the 25 patients. At the end of this process, the PMD associated with each visual field was obtained from the patient records. The minimal change in PMD that best discriminated between those who did and did not have a clinically significant change in their visual field examination was estimated using receiver operating characteristic curve analysis. This analysis revealed that a change of 1.3 dB yielded a sensitivity of 79% (i.e., 15/19 patients who had a clinically significant change also had a change in PMD of .1.3 dB) and a specificity of 83% (i.e., 5/6 patients who did not have a clinically significant change also had a change in PMD of #1.3 dB). Statistical Analysis The primary analyses will be performed according to the intention-to-treat principle and will include all randomized participants. The primary outcome variable is the change from baseline to month 6 in PMD in the eye with the most severe initial visual loss at baseline. The primary statistical analysis will involve fitting an analysis of covariance model with treatment group as the factor of interest, study site as a stratification factor, and baseline PMD and papilledema grade as covariates. This model will be used to calculate a 95% confidence interval for the adjusted treatment group difference in mean response (treatment effect) at month 6, and a t test will be performed to compare the adjusted treatment group means using a 2-tailed significance level of 5%. Similar models will be used to analyze changes from baseline to months 1, 2, 3, and 4.5. These will be considered to be secondary analyses. Missing data will be imputed using regression-based multiple imputation models. This will be applied using a regression-based imputation model (34). For participants with complete data up to a particular visit, a multiple regres-sion model will be fit that includes the outcome at that visit as the dependent variable and outcomes at previous visits, treatment group, study site, and papilledema grade as inde-pendent variables. Separate models will be similarly con-structed for each visit. Using these regression models, a missing value for a participant at a particular visit will be imputed as a draw from the predictive distribution given the outcomes at previous visits (some possibly imputed), treatment group, center, and papilledema grade. This will be done sequentially starting with the baseline visit and ending with the month 6 visit. This process will be imple-mented 100 times, resulting in 100 complete analysis data sets. The analyses will be performed separately for each of the 100 complete analysis data sets, and the results will be combined into 1 multiple imputation inference (estimated treatment effect and associated confidence interval and P value) (35). This approach is appropriate for data sets that have a monotone missing data pattern. If the data set does not have this pattern, the monotone data augmentation method using Markov chain Monte Carlo methods (36,37) will be used to impute the small amount of missing data that is required to make the missing data pattern monotone before applying the multiple imputation algorithm described above. A secondary strategy for analysis will involve the use of a repeated-measures analysis of covariance model, that is, the so-called mixed model repeated measures, or MMRM, analysis strategy (38) that includes treatment group as the factor of interest, study site as a stratification factor, and baseline PMD and papilledema grade as covariates. The model will also include terms for visit (categorical), the interaction between baseline PMD and visit, and the inter-action between treatment group and visit. The covariance matrix for the within-participant observations will be mod-eled using an unstructured pattern. Interactions between treatment group and selected baseline variables (age; race/ethnicity; baseline PMD; papilledema grade; weight change in the previous 6 months; and constant visual loss, scotoma, and color vision loss) will be examined separately by adding the appropriate interac-tion term to the primary statistical model. Treatment effects on secondary outcome variables for efficacy that are expected to be approximately normally distributed (perhaps after data transformation) will be analyzed as described above for the primary outcome variable. For presence of headache, logistic regression (with multiple imputation for missing data) will be used to assess treatment effects. An additional analysis of the PMD outcome will be performed that includes all eyes that satisfy the criterion of having a baseline value between 22 and 27 dB. The MMRM strategy of analysis as described above will be used, except that it will also accommodate the nonzero correlation between the within-subject outcomes for the 2 eyes, where applicable. Study Visits To view a schedule of activities, view Supplemental Digital Content, Table E1, http://links.lww.com/WNO/A96. Screening Evaluation The screening process occurred at 1 or more visits over 7 days. Each treatment trial candidate had general medical Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 113 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. and neurologic examinations and history, height, weight, blood pressure, waist circumference, urine pregnancy test in women, complete blood count, blood chemistry panel, and blood for vitamin A and genetics studies. Participants underwent an ophthalmic evaluation, perimetry, fundus photography, and QOL assessments. An magnetic resonance imaging of the brain (within 2 months of study entry) and a diagnostic LP including opening pressure measurement with CSF cell count, glucose, and protein measurements were performed as part of routine clinical care. However, the CSF from the LP performed for the diagnosis was used for the vitamin A and sodium investigational studies when available. All enrollees were offered an initial evaluation by telephone within a week of randomization by a study weight loss counselor at NYONRC to determine the appropriate dietary intervention. Subsequent Visits and Monitoring Eligible consenting individuals completed the randomiza-tion process and were dispensed study medication. Follow-up visits occurred at months 1, 2, 3, 4.5, 6, 9, and 12 within a 7-day window on either side of the target date. Follow-up was performed by telephone at months 7 and 8. At follow-up, subjects were queried about their interim medical history and headache status, adverse experiences, and concomitant medications used. Their visits included assessment of vital signs (including height and weight) and waist circumference, ophthalmic examination, perimetry, and fundus photographs. Study drug was dispensed, and adherence/accountability was assessed at all visits. Electro-lytes were checked at baseline and every 2 weeks during dosage escalation and at 1, 3, and 6 months. Appropriate potassium supplementation was given for hypokalemia. At the month 6 visit, participants also had a urine sodium analysis, blood sample for vitamin A studies and storage, an additional visual field examination, QOL assessments, and Patient-Centered Assessment and Counseling for Exercise Current Physical Status Questionnaire. All participants were asked to undergo a repeat LP at 6 months after the month 6 visual field testing. Treatment Failures and Premature Withdrawals Conditions for premature withdrawal from the study of randomized participants included withdrawal of consent, a request for withdrawal by the Study Director or primary care physician, pregnancy, a major protocol deviation, loss to follow-up, worsening of another pre-existing disease, intercurrent illness that interfered with the use of study medication or possibly altered the course of IIH, a major and clinically significant alteration in laboratory values after beginning the study drug, other serious safety concerns, or meeting criteria for treatment failure. The study drug was discontinued immediately in women who became pregnant. All randomized participants who discontinued taking study drug because of pregnancy or other medical conditions were nevertheless asked to return for follow-up visits; the outcomes of all pregnancies were ascertained after delivery. Possible treatment failure at follow-up was determined as a function of the PMD at baseline as follows: if the baseline average PMD ranged from 22.0 to 23.5 dB and visual function worsened more than 2 dB PMD from this baseline average in either eye, then possible treatment failure existed. If the baseline average PMD was between 23.5 and 27 dB and the PMD declined more than 3 dB from this baseline average in either eye, then possible treatment failure existed. When the VFRC suspected possible treatment failure, the site PI was notified by the DCBC within 24 hours and the worsening was confirmed with repeat visual field examination at least 1 hour but within 4 days after the original visual field examination. If the worsened PMD was confirmed, the case was referred to the Study Director to ascertain whether treatment failure had occurred. After reviewing all clinical data from the DCBC, PRC, and VFRC, the Study Director determined whether the worsening of the visual field was likely due to uncontrolled intracranial pressure and progression of IIH. If he was unable to make this determination confidently, the data, including visual fields and fundus photographs, were sent to the Adjudication Committee that consisted of 4 neuro-ophthalmologists. The Chair of the Adjudication Committee also reviewed all cases determined to be treatment failures by the Study Director for additional confirmation. After confir-mation was made, the study participant was classified as a treatment failure and was removed from the double-masked phase of the trial. Subsequent treatment decisions were made by the treating physician. These individuals were encouraged to return for study visits at their set times for the full follow-up period (up to 4 years). If the end point of treatment failure was not confirmed, the subject continued to be followed in the trial on study medication. Enrollment and Long-term Follow-up One hundred sixty-five consenting participants enrolled at 38 sites between March 17, 2010, and November 27, 2012, and month 6 visits were completed by June 15, 2013. Annual follow-up examinations will continue for a total of 5 years to determine the visual prognosis, assess headache status over time, and ascertain the long-term effect of the dietary and lifestyle intervention on weight. CASE-CONTROL STUDY TO INVESTIGATE THE ETIOLOGY OF IIH Objectives In addition to the treatment trial, secondary objectives of the IIHTT were to determine 1) the serum and CSF levels of potential mediators of IIH suggested by the genotyping at single-nucleotide polymorphisms (SNPs) contained within genes encoding molecules likely to be involved in 114 Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. the etiology of IIH; 2) differences in vitamin A and other factors (e.g., leptin, grehlin) related to metabolism in subjects and controls; and 3) hormone levels related to obesity and to gender in IIH cases and controls using the results of the genetic testing. Study Population Controls without IIH for genetic testing were matched as closely as possible to the participants in the randomized trial ("cases"). Each control was of age 18-60 years, had a body mass index .30 kg/m2, and was the same gender and the same reported race/ethnicity as a trial participant enrolled at the site. Individuals with a history of IIH or evidence of papilledema by direct ophthalmoscopy were ineligible to serve as controls. Controls had 1 study visit to measure their height, weight, and waist circumference; complete the Berlin Questionnaire for Sleep Apnea; and submit a blood sample for genetic testing, vitamin A studies, and storage. Outcome Measures Genetic Markers Associated with IIH Rationale In addition to the treatment trial, the IIHTT included exploratory studies in this large cohort of patients to gain additional insight into possible causes of the disorder, including genetic factors, obesity hormones, and abnormal vitamin A metabolism (39-50). Candidate genes for a role in the patho-genesis of IIH will be investigated by conducting a nested matched case-control genetic association study in which the frequencies of intragenic SNP alleles will be compared between treatment trial participants and obese controls without IIH. Procedure The candidate genes to be screened in the IIHTT were selected because of their role in sodium homeostasis, vitamin A or fat metabolism, or as cellular mediators of sex hormone signaling. Data from the HapMap (51), UCSC (52), and dbSNP (53) databases were used to iden-tify informative SNPs for screening. As a first step, we will genotype samples received from approximately half of all IIH subjects and non-IIH controls at SNPs located within each of the candidate genes at a ,20 kb average density. One hundred ninety-two SNPs associated with 43 candidate genes will be examined. A minimum of 2 SNPs in the coding region and 1 SNP in the upstream regulatory region will be studied. Priority will be given to SNPs that represent changes in amino acid sequence (coding region only) that do not appear to be specific for any ethnic population and that are present with a minor allele frequency of .5%, with higher values more desirable. In the second step, variants that show a statistically significant difference (P , 0.05) between cases and controls (by Fisher exact test for rare mutations or chi-square analysis for common polymorphisms) will be validated in the second group of specimens from cases and controls. Retinol and Vitamin A Metabolism Retinol (vitamin A) levels were measured in both serum (151 IIH cases and 17 non-IIH controls) and CSF using high-performance liquid chromatography, also allowing measurement of alpha- and gamma-tocopherol and carotenoids (alpha- and beta-carotenes, lycopene, and beta-cryptoxanthin) concentrations in the serum. Retinol-binding protein levels were determined by radioimmunoassay in serum (n = 161) and CSF (n = 107) (54). The active vitamin A metabolite, all-trans-retinoic acid, was measured in both serum (n = 174) and CSF (n = 125) using liquid chro-matography- tandem mass spectrometry. Plasma/CSF pairs were available for analysis in approximately 2/3 of cases of IIH. OCT ANCILLARY STUDY Objective An ancillary study was added to establish whether high-definition OCT can provide a continuous measure of structural changes of papilledema in the optic nerve head and retina that correlate with Frisén grading, visual field deficits, and low-contrast visual acuity. Study Population and Procedures Participants at 24 sites with availability of the specific high-definition OCT machine (Cirrus; Zeiss-Meditc, Inc., Jena, Germany) had volume scans of the optic nerve head and macula regions at study entry, 1, 3, and 6 months to measure the optic nerve head volume, peripapillary retinal nerve fiber layer and total retina thickness, optic nerve head shape, macula edema, and ganglion cell layer thickness. Low-contrast (2.5%) Sloan visual acuity was tested in both eyes at each study visit in which the OCT was performed. Seventy-six percent of all treatment trial participants took part in the ancillary OCT study. STUDY ORGANIZATION Organizational Structure The study was conceived and designed under the auspices of the NORDIC (see Supplemental Digital Content, Figure E1, http://links.lww.com/WNO/A95). The National Eye Insti-tute provided support for the NORDIC Headquarters and for the DCBC. The NORDIC Chair's Office was responsible for aspects of study site management, including site selection, contracts and budget, study committee and investigator meet-ings, and site monitoring visits. NORDIC maintained a Web site that included a message board for participants. The DCBC at the University of Rochester developed, revised, and distributed the study operations manual and case report forms. The DCBC collected all the data electronically by means of an Internet connection to a secure portal and was responsible for data monitoring and data analysis. The Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 115 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. VFRC and OCT Reading Center (University of California at Davis), the PRC (University of Rochester), and CMSU (University of Rochester) reported to the DCBC. The Study Steering Committee, chaired by the Study Director, included the Study Co-Directors, Study Biostatis-tician, Clinical Monitor, NEI Representative, NORDIC Network Chair and Regional Project Coordinator, DCBC Director and Project Manager, PIs of the reading centers, 1 site investigator, and 1 site coordinator. The committee instituted conference calls biweekly throughout the study duration and scheduled in-person meetings twice yearly. The IIHTT adjudication committee, composed of neuro-ophthalmologists, was established to review possible treatment failure cases. An independent DSMB established a charter specifying once-yearly in-person meeting and once-yearly teleconference. CONCLUSIONS The IIHTT is the first randomized, placebo-controlled double-masked study to assess treatment strategies and investigate potential etiologies of IIH in patients with mild visual loss. Baseline data will be reported separately and results related to the primary study end point are anticipated to be announced in the Spring of 2014. ACKNOWLEDGMENTS The authors gratefully acknowledge the staff at the University of Rochester Clinical Trials Coordination Center for their guidance and expertise; the University of Rochester Research Subjects Review Board members; the individuals with IIH who entered the screening process, those who ultimately enrolled as study participants, and those who kindly consented to volunteer as control subjects. Please refer to the attached list of the IIHTT Study Group members (see Supplemental Digital Content, Table E2, http://links.lww.com/WNO/A97). REFERENCES 1. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 1991;9:73-95. 2. Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45:875-877. 3. Garrett JH, Corbett JJ, Braswell R. The incidence of idiopathic intracranial hypertension in Mississippi. Paper presented at: The North American Neuro-ophthalmology Society; 2004; Orlando, FL. 4. Wall MW, George D. Idiopathic intracranial hypertension (pseudotumor cerebri). A prospective study of 50 patients. Brain. 1991;114:155-180. 5. Lueck C, McIlwaine G. Interventions for idiopathic intracranial hypertension. Cochrane Database Syst Rev. 2005;CD003434. 6. Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5:55-56. 7. Wall M, White WN. Asymmetric papilledema in idiopathic intracranial hypertension: prospective interocular comparison of sensory visual function. Invest Ophthalmol Vis Sci. 1998;39:134-142. 8. Kleinschmidt JJ, Digre KB, Hanover R. Idiopathic intracranial hypertension: relationship to depression, anxiety and quality of life. Neurology. 2000;54:319-324. 9. Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, Biousse V, Lee AG, Wall M, Kardon R, Acierno MD, Corbett JJ, Maguire MG, Balcer LJ. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension (pseudotumor cerebri). Am J Ophthalmol. 2007;143:635-641. 10. Kammerdiener L, Friedman DI. Patient interviews and focus groups to determine outcome measures for idiopathic intracranial hypertension. Paper presented at: North American Neuro-ophthalmology Society; 2010; Tucson, AZ. 11. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short- Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263. 12. Kosinski M, Bayliss MS, Bjorner JB, Ware JE Jr, Garber WH, Batenhorst A, Cady R, Dahlöf CG, Dowson A, Tepper S. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963-974. 13. Cleary PA, Beck RW, Anderson MM Jr, Kenny DJ, Backlund JY, Gilbert PR. Design, methods and conduct of the Optic Neuritis Treatment Trial. Control Clin Trials. 1993;14:123-142. 14. Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45:13-18. 15. McCarthy KD, Reed DJ. The effect of acetazolamide and furosemide on CSF production and choroid plexus carbonic anhydrase activity. J Pharmacol Exp Ther. 1974;189:194-200. 16. Lubow M, Kuhr L. Pseudotumor cerebri: comments on practical management. In: Glaser JS, ed. Neuro-ophthalmology. Symposium of the University of Miami and the Bascom Palmer Eye Institute. Vol IX. Saint Louis, MO: The C.V. Mosby Company; 1977:199-206. 17. Gücer G, Viernstein L. Long-term intracranial pressure recording in the management of pseudotumor cerebri. J Neurosurg. 1978;49:256-263. 18. Tomsak RL, Niffenegger AS, Remler BF. Treatment of pseudotumor cerebri with Diamox (acetazolamide). J Clin Neuroophthalamol. 1988;8:93-98. 19. Epstein DL, Grant WM. Carbonic anhydrase inhibitor side effects. Serum chemical analysis. Arch Ophthalmol. 1977;95:1378-1382. 20. Kupersmith MJ, Gamell L, Turbin R, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50:1094-1098. 21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension. Ophthalmology. 1998;105:2313- 2317. 22. Newborg B. Pseudotumor cerebri treated by rice reduction diet. Arch Intern Med. 1974;133:802-807. 23. Jeffery RW, Sherwood NE, Brelje K, Pronk NP, Boyle R, Boucher JL, Hase K. Mail and phone interventions for weight loss in a managed-care setting: weigh-to-be one-year outcomes. Int J Obes Relat Metab Disord. 2003;27:1584-1592. 24. Sherwood NE, Jeffery RW, Pronk NP, Boucher JL, Hanson A, Boyle R, Brelje K, Hase K, Chen V. Mail and phone interventions for weight loss in a managed-care setting: weigh-to- be 2-year outcomes. Int J Obes (Lond). 2006;30:1565- 1573. 25. Brownell KD. LEARN Program for Weight Management, 10th edition. Euless, TX: American Health Publishing Company, 2000. 26. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241-247. 27. Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537-549. 28. Friedman DI, Streeten DHP. Idiopathic intracranial hypertension and orthostatic edema may share a common pathogenesis. Neurology. 1998;50:1099-1104. 29. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. American Heart Association Dietary approaches to prevent and treat hypertension: a scientific statement from 116 Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. the American Heart Association. Hypertension. 2006;47: 296-308. 30. National Cholesterol Education Program. The report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult treatment panel III). Circulation. 2002;106:3143-3421. 31. Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Low-energy-density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006;106:1172-1180. 32. Wansink B, Chandon P. Meal size, not body size, explains errors in estimating the calorie content of meals. Ann Intern Med. 2006;145:326-332. 33. Wansink B. Environmental factors that increase the food intake and consumption volume of unknowing consumers. Annu Rev Nutr. 2004;24:455-479. 34. Little R, Yau L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics. 1996;52:1324-1333. 35. Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton, FL: Chapman and Hall/CRC, 1997. 36. Li KH. Imputation using Markov chains. J Statist Comput Simul. 1988;30:57-79. 37. Liu C. Bartlett's decomposition of the posterior distribution of the covariance for normal monotone ignorable missing data. J Mult Anal. 1993;46:198-206. 38. Molenberghs G, Thijs H, Jansen I, Beunckens C, Kenward MG, Mallinckrodt C, Carroll RJ. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5:445- 464. 39. Buchheit W, Burton C, Haag B, Shaw D. Papilledema and idiopathic intracranial hypertension. Report of familial occurrence. N Engl J Med. 1969;280:938-942. 40. Coffey CE, Ross DR, Massey EW, Olanow CW. Familial benign intracranial hypertension and depression. Can J Neurol Sci. 1982;9:45-47. 41. Gardner K, Cox T, Digre KB. Idiopathic intracranial hypertension associated with tetracycline use in fraternal twins: case reports and review. Neurology. 1995;45:6-10. 42. Howe J, Saunders M, Clarke P. Familial benign intracranial hypertension. Acta Neurochir (Wien). 1973;29:173-175. 43. Kharode C, McAbee G, Sherman J, Kaufman M. Familial intracranial hypertension: report of a case and review of the literature. J Child Neurol. 1992;7:196-198. 44. Traviesa DC, Schwartzman RJ, Glaser JS, Savino P. Familial benign intracranial hypertension. J Neurol Neurosurg Psychiatry. 1976;39:420-423. 45. Lombaert A, Carton H. Benign intracranial hypertension due to A-hypervitaminosis in adults and adolescents. Eur Neurol. 1976;14:340-350. 46. Marie J, See G. Acute hypervitaminosis A of the infant. Its clinical manifestation with benign acute hydrocephaly and pronounced bulge of the fontanel. A clinical and biologic study. AMA Am J Dis Child. 1954;73:731-736. 47. Gerber A, Raab AR. Vitamin A poisoning in adults. With description of a case. Am J Med. 1954;16:729-745. 48. Jacobson DM, Berg R, Wall M, Digre KB, Corbett JJ, Ellefson RD. Serum vitamin A concentration is elevated in idiopathic intracranial hypertension. Neurology. 1999;53:1114-1118. 49. Selhorst JB, Kulkantrakorn K, Corbett JJ, Leira EC, Chung SM. Retinol-binding protein in idiopathic intracranial hypertension. J Neuroophthalmol. 2000;20:250-252. 50. Warner JEA, Bernstein PS, Yemelyanov A, Alder SC, Farnsworth ST, Digre KB. Vitamin A in the cerebrospinal fluid of patients with and without idiopathic intracranial hypertension. Ann Neurol. 2002;52:647-650. 51. International HapMap Consortium. The International HapMap Project. Nature. 2003;426:780-796. 52. Karolchik D, Baertsch R, Diekhans M, Furey TS, Hinrichs A, Lu YT, Roskin KM, Schwartz M, Sugnet CW, Thomas DJ, Weber RJ, Haussler D, Kent WJ. The UCSC Genome Browser database. Nucleic Acids Res. 2003;31:51-54. 53. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308-311. 54. Blaner WS. Radioimmunoassays for retinol-binding protein, cellular retinol-binding protein, and cellular retinoic acid-binding protein. Methods Enzymol. 1990;189:270-281. Friedman et al: J Neuro-Ophthalmol 2014; 34: 107-117 117 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |