| OCR Text |
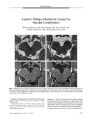
Show ORIGINAL CONTRIBUTION Effect of Age on the Pupillomotor Field Ruediger Schmid, MD, FEBO, Per Ceurremans, Holger Luedtke, Barbara J. Wilhelm, MD, and Helmut M. Wilhelm, MD Abstract: To differentiate physiologic variation from visual field loss with pupillomotor perimetry, the effect of age on the normal pupillomotor field must be known. Given the absence of reported data, the authors aimed to analyze the effect of age on the pupillomotor field as measured with light stimuli of different properties. Subjects consisted of 23 healthy volunteers aged 20 to 28 years (" younger subjects") and 20 healthy volunteers aged 50 to 67 years (" older subjects"). Within a field of 20°, a sequence of 25 focal light stimuli was performed repeatedly on a monitor. The pupil light reflex ( PLR) was recorded to stimuli of different diameter and luminance under mesopic conditions. The mean amplitude of the PLR was calculated for each stimulus location and condition. Increasing stimulus luminance or size caused a larger PLR amplitude and a steeper decline of the PLR amplitude from the center to the periphery of the pupillomotor field. The older subjects had reduced mean PLR amplitude with a less pronounced decrease of PLR amplitude toward the field periphery. For the peripheral locations, the largest PLR amplitude was found in the temporal superior quadrants. There was considerable intra- individual test- retest variation in PLR amplitudes in younger and older subjects. The PLR is markedly reduced in older compared with younger subjects. Older subjects have a relatively less pronounced central peak of sensitivity. There are intra-individual test- retest variations in PLR amplitude and asymmetries in sensitivity within the normal pupillomotor field at any age. These findings must be considered in interpreting the results of pupillomotor perimetry. ( JNeuvo- Ophthalmol 2004; 24: 228- 234) University Eye Hospital, Department of Neuro- ophfhalmology and Pathophysiology of Vision, Tuebingen, Germany. Supported by Fortuene 531, a grant to the University of Tuebingen, Germany. Address correspondence to Barbara J. Wilhelm, MD, Abteilung fuer Neuroophfhalmologie und Pathophysiologie des Sehens, Schleichstr. 12- 16, D- 72076, Tuebingen, Germany; E- mail: barbara. wilhelm@ uni- tuebingen. de Conventional perimetry is limited by the subjectivity of patient response, by poor fixation, and, in some cases, by poor understanding of the task. Pupillomotor perimetry is an objective method of investigating the visual field ( 1- 3) that makes use of the pupil light reflex ( PLR). It requires less patient attention, does not show any learning effect, and can indicate psychogenic visual field loss. In pupillomotor perimetry, a small light stimulus is presented at several discrete locations in the visual field. The amplitude of the pupil's contraction to this stimulus is measured by means of infrared video- based pupillography. The mean amplitudes at the different stimulus locations result in the patient's pupillomotor field. In areas of visual field loss, the PLR is reduced compared with the PLR elicited within field areas of normal visual function. To judge the pupillomotor field of a patient, the profile in retinal sensitivity for the PLR in normal eyes must be known. Further, knowledge of the dependency of the pupillomotor field profile on age is crucial to differentiate physiological depressions of PLR sensitivity from field loss. To our knowledge, the impact of age on the pupillomotor field has not been reported. We therefore investigated younger and older healthy subjects by pupillomotor perimetry with different kinds of light stimuli. The aim of the study was to determine the impact of age on the pupillomotor field and the influence of stimulus properties on the field profile in each age group. METHODS Subjects We included 23 subjects aged 19 to 28 years ( classified as " young") and 20 subjects aged from 50 to 67 years (" old"). There were 14 women and 9 men among the younger subjects, and 12 women and 8 men among the older subjects. All were healthy volunteers from whom informed consent had been obtained. The study followed the tenets of the Declaration of Helsinki. All subjects underwent a test of visual acuity, slit lamp and fundus examination, applanation tonometry, and swinging flashlight test. Normal results in all tests were required to enter the study. Bilateral mild lens opacities were allowed in the older group. Ametropia was allowed up to ± 5 diopters spherical 228 JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 Effect of Age on the Pupillomotor Field JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 equivalent. Corrected visual acuity was 20/ 20 or better for the younger and 20/ 25 or better for the older subjects. Pupillography Infrared video- based pupillography was performed with a device described in detail elsewhere ( 1). Light stimuli were generated on a computer screen at a distance of 30 cm from the subject's eye. The luminance across the screen varied by approximately 3%. Before running the test, the monitor had to be switched on for 30 minutes and the subjects had then to adapt to the steady background luminance for 10 minutes. Within a visual field of 20°, a fixed sequence of 25 stimuli was performed four times to cope with intra- individual variation. Stimulus eccentricity was at 0, 5, 11, and 20° ( Fig. 1). The stimulus was varied systematically between diameters of 2, 3, and 4° and luminance between 26 cd/ m2 and 54 cd/ m2 with a monitor background luminance of 1 cd/ m2. The stimulus interval was 2.4 seconds, with stimulus duration of 0.2 seconds. Total testing time was 4 minutes for each kind of stimulus. Only one eye was tested per subject. An infrared- sensitive camera linked to a frame grabber card recorded the direct PLR with a spatial resolution of 0.05 mm. After artefact rejection, the contraction amplitude of the PLR was calculated from each recorded pupil trace. For artefact rejection, a smoothing of the raw data trace was performed and aberrant data were discarded. A pupil response had to occur between 200 and 500 ms after stimulus onset. The slope of the pupil tracing before onset of the light stimulus had to be less than 1 mm/ s. During the light stimulus, the maximum difference between a single value and the mean of the pupil tracing had to be less than 0.3 mm and the superior 157.5° rr4 112.5° 67.5° \\< m° j°° \ 22.5° Wphi 15° 2D° eccentricity temporal nasal FIG. 1. A stimulus pattern showing the four meridians at different angles (" phi") across the pupillomotor field of OS. Based on these meridians, PLR field profiles are presented in Figure 2. slope during the stimulus had to be between 0.7 and 0.9 mm/ second. Another restriction was applied to the whole PLR, requiring the standard deviation of the original curve ( without outliers) and the smoothed curve to be less than 0.12 mm. To detect the minimum, five neighboring values were required to have a typical shape. The empirical values of these restrictions assured high- quality in determination of the PLR. No criterion was applied for the extent of the pupil response to avoid a distorted data distribution. When a discontinuity of the pupil trace ( for example, blinking artefact) occurred during pupil contraction or stimulus presentation, this measurement was discarded. Statistical Analysis The position of the light stimulus in the visual field was defined by the eccentricity and by the angle (" phi") between the x- axis and a straight line connecting fixation mark and stimulus location. We could evaluate 93% of all stimulus responses. For evaluation, the OD results were transformed so that all eyes could be considered as the OS. A commercial software package ( JMP 3.2.5; SAS Institute Inc, Cary, NC) was used for the statistics. A linear model was calculated with the contraction amplitude as response variable ( ANOVA). The following factors were tested: age group, stimulus luminance, stimulus diameter, and, as random effect, the subject, nested within the age group. As co-variables, we included eccentricity and pupil resting size. The trigonometric functions of phi, sine phi, and cosine phi were used for the model as pairwise interaction terms eccentricity/ sine phi and eccentricity/ cosine phi, because phi, sine phi, and cosine phi are not steady functions at 0° ( center of the visual field). Other pairwise interactions of the model were luminance/ diameter, eccentricity/ eccentricity, luminance/ eccentricity, diameter/ eccentricity, age group/ eccentricity, age group/ luminance, age group/ diameter. Our model results in a continuous profile of the central visual field. RESULTS With increasing stimulus luminance and size, there was a larger pupil response across the pupillomotor field with a steeper decline of the PLR from the center to the periphery. Increasing stimulus size and luminance had a synergistic effect on raising the PLR amplitude. The extent of decline of the PLR amplitude from the center to the periphery of the field varied according to the angle phi. Older subjects had an overall reduced mean PLR amplitude. Increasing stimulus luminance or size resulted in a significantly larger increase of the mean PLR amplitude among the younger than the older subjects. The overall decline of PLR amplitudes from the center to the periphery was more pronounced for the young group ( Table 1). 229 JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 Schmid et al TABLE 1. Analysis of variance of pupillary light reflex Source Age group Stimulus size Stimulus luminance Stimulus size* stimulus luminance Eccentricity Eccentricity squared Eccentricity* sine phi Eccentricity* cosine phi Stimulus size* eccentricity Stimulus luminance* eccentricity Age group* stimulus size Age group* stimulus luminance Age group * eccentricity Pupil resting size Subject ( age group) Sum of squares 11.4 51.2 48.1 6.39 30.2 12.1 11.2 28.7 3.47 4.12 2.19 2.66 0.28 0.80 403 * marks interaction of factors. The random effect " subject" is amplitudes in younger and older Mean square 11.4 25.6 48.1 3.19 30.2 12.1 11.2 28.7 1.73 4.12 1.09 2.66 0.28 0.80 9.83 nested within the effect Degrees healthy subjects of freedom 1 2 1 2 1 1 1 1 2 1 2 1 1 1 41 ' age group," indicated F ratio 10.22 1,239 2,328 154.5 1,465 585.1 544.2 1,389 84.01 199.3 53.10 129.0 14.01 39.05 475.5 by parentheses. P 0.0026 0.0000 0.0000 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 < 0.0001 0.0002 < 0.0001 0.0000 The raw data mean values and standard deviations for different eccentricities of the field are shown in Table 2. As an example, cross sections of the pupillomotor field at different angles for a stimulus diameter of 3° are shown for both age groups in Figure 2. For both age groups, the temporal pupillomotor field was more sensitive than the nasal field. The superior field was more sensitive than the inferior field. Based on the model, the amplitudes of the PTR across the pupillomotor field had a maximum at an angle of 148° ( within the temporal superior quadrant). A three-dimensional plot of the modeled pupillomotor field is shown as an example in Figure 3. Intra- individual variation was considerable. The single PTR amplitudes of one younger and one older subject are shown in Figure 4. DISCUSSION Stimulus Properties In this study, the higher stimulus intensities better revealed the differences in sensitivity of the pupillomotor field, despite the inevitable increase in local retinal light scatter. However, the highest stimulus intensity used here ( 54 cd/ m2) still was relatively dim. With a stimulus luminance of 26 cd/ m2 and a stimulus diameter of 3°, the PTR was close to the noise level for most peripheral stimulus locations. For the 2° stimulus, this was even true for some peripheral locations when a luminance of 54 cd/ m2 was applied. Stimulus intensity increases with both size and luminance. For the pupillary system, larger receptive fields of the retinal ganglion cells and more extensive spatial sum- TABLE 2. of stimulus Size Lum 2° 26 54 3° 26 54 4° 26 54 Mean pupillary light reflex amplitudes size and luminance 0° Ampl 0.35 0.50 0.46 0.68 0.55 0.81 SD 0.16 0.22 0.22 0.21 0.18 0.31 5° Ampl 0.22 0.39 0.36 0.54 0.43 0.64 Young SD 0.09 0.16 0.12 0.18 0.15 0.21 11 Ampl 0.16 0.29 0.26 0.43 0.33 0.53 in young and old subjects at four eccentricities and for different combinations SD 0.07 0.14 0.11 0.15 0.13 0.20 20 Ampl 0.14 0.24 0.22 0.34 0.27 0.46 SD 0.07 0.12 0.09 0.13 0.12 0.18 0° Ampl 0.26 0.39 0.35 0.54 0.43 0.69 SD 0.18 0.20 0.20 0.22 0.22 0.30 5C Ampl 0.17 0.28 0.25 0.42 0.33 0.52 Old SD 0.14 0.18 0.17 0.18 0.18 0.21 11 Ampl 0.12 0.18 0.17 0.30 0.23 0.40 ; SD 0.11 0.14 0.13 0.17 0.15 0.18 20° Ampl SD 0.12 0.09 0.15 0.12 0.14 0.12 0.23 0.14 0.18 0.13 0.32 0.15 Lum, luminance; Ampl, amplitude. 230 © 2004 Lippincott Williams & Wilkins Effect of Age on the Pupillomotor Field JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 B Kc « rf TT iT IT T. ! 1 V I 1- T T E Tr I S, F* * * » * \ VM\ \ fH** t TT 4 T i IT [ i I IT 1 • 1 IT 1 &. t*** tr\ N I TT IT i- i I- TT TT I j} [[ l 1 T{ I • < - - . • fc « * i:* n T iT I ) TT Tr T k 1 1 [] i il - i 0 » 5. JO « i « « tt; » iri 1 SfcfJrV •• w » T TT i l - T TT TT il \ i^{ { J Is . T I: I T \ i1 IT IT IT I| 1 [[ i i t; t « » : ff] T ll 1 FIG. 2. The cross- sections of the pupillomotor field with a stimulus diameter of 3°. Mean PLR amplitudes ± SD ( raw data) are shown for each stimulus location at a certain cross- section for the young and for the old group. The x- axis runs from the inferior field toward the superior field. A: The field profile at the four different angles (" phi") indicates a stimulus luminance of 26 cd/ m2. B: The field profile indicates a stimulus luminance of 54 cd/ m2. 231 JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 Schmid et al 0 . 8.: amplitude ( nnm) upertor 0 - 20 temporal - 10 10 nasal 2 0 FIG. 3. Three- dimensional plot of the pupillomotor field for the younger subjects exposed to a stimulus luminance of 54 cd/ m2 and a stimulus diameter of 3°. mation are assumed compared with the visual system ( 4). Thus, the stimulus of 4° still seems to be suitable, but a stimulus of 3° is expected to ensure a better spatial resolution. For the measurement settings used here, a stimulus of 54 cd/ m2 and a diameter of 3 or 4° elicited PLR amplitudes sufficiently above noise level. In pupillography, noise is found at two levels. First, a certain spread of original data occurs when recording the pupillary movement. Second, it is well- known that the pupil always performs minor movements as a function of the dynamic pupil steady- state diameter. These movements represent light- induced oscillations and underlie emotional and autonomic nervous system activities. They increase in amplitude with growing sleepiness. Age Dependency of the Pupillomotor Field The age of the subject had a clear impact on the pupillomotor field. For the older subjects, the amplitudes of the PLR across the pupillomotor field were globally reduced. The question arises whether this is caused by reduced responsiveness of the pupillomotor system with age, neuronal cell loss, increased media opacities, or decreased pupil size. The known decline of pupil size with age ( 5), which was found in our study population, will reduce retinal illumination to some extent. The difference in mean pupil resting size between both groups of subjects will reduce the retinal light flux for the old subjects. Because the PLR amplitude is proportional to the logarithm of the retinal light flux in a suprathreshold range, the PLR will be reduced. However, a smaller pupil will increase retinal sensitivity to a certain extent because of adaptation. Hence, it is difficult to weigh both effects on the PLR. In the present experiment, a small relationship was found between pupil resting size and contraction amplitude, but it is not possible to separate this statistically from the age effect already discussed. Iris mechanics of the smaller pupil ( 6) did not have a substantial impact on the pupil's contraction here because the mechanical limit generally was not reached. A decrease in efficiency of the focal light entering the eye by cataract or vitreous clouding was unlikely to have caused a reduction of the PLR in the older subjects, as their visual acuity still was at least 20/ 25. It is well- established that mild media opacities generally do not reduce the PLR. With the swinging flashlight test, they may even increase the PLR in the affected eye. However, reduced neuronal sensitivity may be the principal mechanism behind the present findings. A loss of neuronal population has been described at most levels in the aged human visual system ( 7). Most reports indicate that axon density of the optic nerve decreases significantly with age ( 8,9). Comparison to Age- Related Findings in Psychophysics and Electrophysiology A decreased retinal sensitivity in older subjects has been shown for the normal central visual field by different methods of perimetry ( 10- 15). An age- related decrease in differential light sensitivity, as well as in flicker sensitivity for higher frequencies, have been seen mainly in the superior parts of the field ( 11- 13,16). In our study, the decrease of sensitivity from the center to the periphery was less pronounced in the older subjects. One could attribute this to light scatter because of cataract or vitreous clouding ( 17). If so, the more peripheral stimuli might have been relatively more effective because of some scattered light reaching the fovea. Yet these differences were subtle. For the visual field, most studies have reported a steeper decline of differential light sensitivity toward the periphery with age ( 13,14,16). This corresponds to a more accelerated loss of peripheral photoreceptors and ganglion cells compared with central cones in aging ( 18,19). In the multifocal electroretinogram ( mERG), an age-related loss of amplitude and an increase in implicit time has occurred without major changes in the topography of implicit times ( 20). In pattern ERG, consistently lower amplitudes in older subjects have been demonstrated ( 21,22). These could not be explained by differences in retinal image quality but were attributed to age- related neurophysi- 232 © 2004 Lippincott Williams & Wilkins Effect of Age on the Pupillomotor Field JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 E E 54 coVm* size 3 phi = 157.5° x J ID 10 ( young) ID 30 ( old) i l l I - 20 nasal inferior - 15 - 10 - 5 0 5 10 15 20 Eccentricity [*] temporal superior FIG. 4 . A cross- section of the pupillomotor field at an angle of 157.5°. It shows the single PLR amplitudes for one younger subject and for one older subject with a stimulus luminance of 54 cd/ m2 and a stimulus diameter of 3°. The substantial intra- individual test- retest variation in PLR amplitudes is apparent. ological changes in the retina ( 21). In full- field electroret-inogram, decreasing cone and rod b- wave amplitudes have been found with age, whereas an increase in implicit time is disputed ( 23,24). Age- related increases in VEP latency seem to depend on the properties of the stimulus. Postretinal mechanisms have been postulated as an explanation for these phenomena ( 21). With pupillomotor perimetry, the entire visual system is investigated ( 2,25,26). The neuronal mechanisms of the PLR might be different from those tested by psychophysics or electrophysiology. Characteristics of the Pupillomotor and Visual Field Profile There are no reported major inter- eye differences in the retinal sensitivity for the PLR in the same individual ( 27). Therefore, we investigated the pupillomotor field OD or OS by arbitrarily transforming the results into a field OS. As expected, there was an increase of the mean PLR with increasing stimulus intensity. For both groups of subjects and for all stimulus properties, the asymmetries in sensitivity for the normal pupillomotor field were subtle and showed a similar general profile. There was a peak of sensitivity in the center of the field. More peripherally, the greatest pupillomotor sensitivity was found in the superior temporal field and the lowest in the inferior nasal field, consistent with previous findings ( 1,3). These results are only partly in accord with asymmetries found in the normal perceptive visual field. A higher sensitivity of the inferior hemifield compared with the superior field has been reported for computer perimetry ( 10,13- 16). The nasal-temporal asymmetry is controversial; most conventional perimetric or psychophysical studies have shown a higher sensitivity for the temporal hemifield ( 14,15,28). Greater sensitivity of the temporal hemifield is expected because of the higher density of photoreceptors and ganglion cells reported for the nasal retina ( 29- 31). The retinal ganglion cells involved in the pupil light reflex may be at least partly different from those integrated within the visual pathway ( 2,4,32). Thus, pupil perimetry seems to be a valuable and differential complement to visual field testing. The normal asymmetries of the pupillomotor field, although statistically significant, are very subtle compared with clinical findings of a pupillomotor field defect ( 2,3,32). Nevertheless, these asymmetries have to be considered when investigating a patient's pupillomotor field. Acknowledgment The authors thank Professor Steven E. Smith, London, England, for valuable comments on the manuscript. REFERENCES 1. Schmid R, Wilhelm B, Wilhelm H. Pupillomotor campimetry in normals. Neuro- ophthalmol 2000; 23: 7- 13 2. Kardon RH. Pupil perimetry. Editorial review. Current Opinion Ophthalmol 1992; 3: 565- 70 3. Kardon RH, Kirkali PA, Thompson HS. Automated pupil perimetry. Pupil field mapping in patients and normal subjects. Ophthalmology 1991; 98: 485- 95 4. Loewenfeld IE. The Pupil: Anatomy, Physiology and Clinical Applications, vol. 1. Detroit: Wayne State University Press; 1993: 142- 6 233 JNeuro- Ophthalmol, Vol. 24, No. 3, 2004 Schmid et al 5. Loewenfeld IE. Pupillary changes related to age. In: Thompson HS, Daroff R, Frisen L, Glaser JS, Sanders MD, eds. Topics in N'euro-ophthalmology Baltimore: Williams and Wilkins; 1979: 124- 50. 6. Loewenfeld IE, Newsome DA. Iris mechanics. I. Influence of pupil size on dynamics of pupillary movements. Am J Ophthalmol 1971: 71: 347- 62 7. Ordy JM, Brizzee KR, Johnson HA. Cellular alterations in visual pathways and the limbic system: Implications for vision and short-term memory. In: Sekuler R, Kline D, Dismukes K, eds. Aging and human visual functions. New York: Alan R. Liss Inc; 1982: 79- 114 8. Balazsi AG, Rootman J, Drance SM, et al. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol 1984; 97: 760- 6 9. Johnson BM, Miao M, Sadun AA. Age- related decline of human optic nerve axon populations. Age 1987; 10: 5- 9. 10. Dietrich TJ, Ata N, Sanger A, et al. Age influences asymmetry in differential luminance sensitivity. In: Wall M, Wild JM, eds. Perimetry Update 1998/ 99, Proceedings of the XHIth International Perimetric Society. The Hague, The Netherlands: Kugler; 1999: 223- 7 11. Casson EJ, Johnson CA, Nelson- Quigg JM. Temporal modulation perimetry: the effects of aging and eccentricity on sensitivity in normals. Invest Ophthalmol Vis Sci 1993; 34: 3096- 102 12. Haas A, Flammer J, Schneider U. Influence of age on the visual fields of normal subjects. Am J Ophthalmol 1986; 101: 199- 203 13. Zulauf M. Normal visual fields measured with Octopus programm Gl. I. Differential light sensitivity at individual test locations. Graefe's Arch Clin Exp Ophthalmol 1994; 232: 509- 15. 14. Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol 1987; 105: 1544- 9 15. Brenton RS, Phelps CD. The normal visual field in the Humphrey Field Analyzer. Ophthalmologica 1986; 193: 56- 74 16. Katz J, Sommer A. Asymmetry and Variation in the Normal Hill of Vision. Arch Ophthalmol 1986; 104: 65- 8 17. Hennelly ML, Barbur JL, Edgar DF, et al. The effect of age on the light scattering characteristics of the eye. Ophthalmic Physiol Opt 1998; 18: 197- 203 18. Gao H, Hollyfield JG. Aging of the Human Retina. Differential Loss of Neurons and Retinal Pigment Epithelial Cells. Invest Ophthalmol Vis Sci 1992; 33: 1- 17 19. Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer's disease and aging. Ann Neurol 1993; 33: 248- 57 20. Langrova H, Seeliger MW, Kretschmann U, et al. Age dependence of Multifocal ERG amplitude and implicit time. In: Kremlacek J, Kuba M, Kubova Z, eds. Abstract book of the 36th ISCEV symposium. Hradec Kralove: ATD Press; 1998: 55 21. Porciatti V, Burr DC, Morrone MC, et al. The effects of aging on the pattern electroretinogram and visual evoked potential in humans. Vision Res 1992; 32: 1199- 209 22. Trick LR. Age- related alterations in retinal function. Doc Ophthalmol 1987; 65: 35- 43 23. Birch DG, Anderson JL. Standardized full- field electroretinogra-phy. Normal values and their variation with age. Arch Ophthalmol 1992; 110: 1571- 6 24. Weleber RG. The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci 1981; 20: 392- 9 25. Cibis GW, Campos EC, Aulhorn E. Pupillary hemiakinesia in su-prageniculate lesions. Arch Ophthalmol 1975; 93: 1322- 7. 26. Hamann KU, Hellner KA, Muller- Jensen A, et al. Videopupillo-graphic and VER investigations in patients with congenital and acquired lesions of the optic radiation. Ophthalmologica 1979; 178: 348- 56. 27. Hong S, Narkiewicz J, Kardon RH. Comparison of pupil perimetry and visual perimetry in normal eyes: decibel sensitivity and variability. Invest Ophthalmol Vis Sci 2001; 42: 957- 65 28. Fahle M, Schmid M. Naso- temporal asymmetry of visual perception and of the visual cortex. Vis Res 1988; 28: 293- 300 29. Curcio CA, Allen KA. Topography of ganglion cells in human retina. JComp Neurol 1990; 300: 5- 25 30. Curcio CA, Sloan KR, Kalina RE, et al. Human photoreceptor topography. J Comp Neurol 1990; 292: 497- 523 31. Jonas JB, Schneider U, Naumann GO. Count and density of human retinal photoreceptors. Graefes Arch Clin Exp Ophthalmol 1992; 230: 505- 10 32. Yoshitomi T, Matsui T, Tanakadate A, et al. Comparison of threshold visual perimetry and objective pupil perimetry in clinical patients. J Neuroophthalmol 1999; 19: 89- 99 234 © 2004 Lippincott Williams & Wilkins |