| OCR Text |
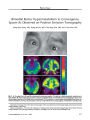
Show ORIGINAL CONTRIBUTION Arachnoid Cyst Causing Third Cranial Nerve Palsy Manifesting as Isolated Internal Ophthalmoplegia and Iris Cholinergic Supersensitivity Lamees Ashker, MD, Joel M. Weinstein, MD, Mark Dias, MD, Paul Kanev, MD, Dan Nguyen, MD, and Dean J. Bonsall, MD, MS, FACS Abstract: An 8-month-old boy presented with anisocoria, a sluggishly reactive right pupil, and cholinergic supersensitivity as the only signs of what proved months later to be compressive third cranial nerve palsy due to an arachnoid cyst. Tonic constriction and dilation, segmental iris sphincter palsy, aberrant regeneration phenomena, ductional deficits, and ptosis were absent. The initial diagnosis was postganglionic internal ophthalmoplegia attrib-uted to a viral ciliary ganglionopathy. Nineteen months later, he had developed an incomitant exodeviation and a supraduction deficit. Brain MRI revealed a mass consistent with an arachnoid cyst compressing the third cranial nerve in the right interpeduncular cistern. Resection of the cyst led to a persistent complete third cranial nerve palsy. This is the second reported case of prolonged internal ophthalmoplegia in a young child as a manifestation of a compressive third cranial nerve palsy. Our patient serves as a reminder that isolated internal ophthalmoplegia with cholinergic supersensitivity is compatible with a preganglionic compressive third nerve lesion, particularly in a young child. (J Neuro-Ophthalmol 2008;28:192-197) Supersensitivity of the iris sphincter to dilute cholinergic agents has long been considered diagnostic of a post-ganglionic third cranial nerve palsy (1). Recent studies have questioned this diagnostic hallmark, having shown that preganglionic third cranial nerve palsy may also demon-strate supersensitivity to dilute cholinergic agents (2-4). This discrepancy in pilocarpine testing is especially relevant in patients who present with isolated pupillary dilation in the absence of other neurologic signs. Tradi-tionally thought to be indicative of benign processes, persistent isolated internal ophthalmoplegia can rarely be the sole manifestation of a third cranial nerve palsy (5-9). We describe a patient in whom an arachnoid cyst compressed the third cranial nerve and produced a pro-longed isolated internal ophthalmoplegia. Our patient is unusual in three respects. First, only one other reported case of compressive third cranial nerve palsy has persisted with-out ptosis or ocular motility dysfunction for longer than 1 year (5). However, in the patient of Wilhelm et al (5) the exact interval between isolated internal ophthalmoplegia and onset of diplopia is uncertain. Second, only one other such case has been reported in a young child, in which the interval between the identification of internal ophthalmoplegia and motility deficits was only 6 weeks (9). Third, there is no report of an arachnoid cyst causing these manifestations. CASE REPORT An 8-month-old boy was first noted to have anisocoria at age 3 months. He had been examined at a local hospital for an episode of lethargy and a viral illness had been diagnosed as the cause of anisocoria. An examining ophthalmologist suggested no further workup. A second ophthalmologist examined him at age 8 months, finding that he was able to follow objects accurately with either eye. Versions were full, and he was orthophoric in all gaze positions with distance and near fixation. In darkness, pupils measured 6 mm in the right eye and 5 mm in the left eye. In room light, pupils measured 6 mm in the right eye and 3 mm in the left eye. There was no afferent pupillary defect. There was no segmental iris sphincter palsy and no vermiform iris movements. Testing for preserved pupil constriction to a near target could not be Departments of Ophthalmology (LA, JMW), Pediatrics (JMW), and Neurosurgery (MD) and Section of Neuroradiology (DN), Penn State University, Milton S. Hershey Medical Center, Hershey, Pennsylvania; Department of Neurosurgery (PK) and Connecticut Children's Medical Center, Tufts University School of Medicine, Boston, Massachusetts; and Department of Ophthalmology (DJB), Cincinnati Children's Hospital, University of Cincinnati, Cincinnati, Ohio. Address correspondence to Joel M. Weinstein, MD, Penn State University, Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; E-mail: jweinstein@hmc.psu.edu 192 J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 performed owing to poor cooperation. There was no ptosis, and there were no signs of aberrant regeneration involving the eyelids or pupil. Results of the remainder of the examination were normal with the exception of mild myopia of 0.50 diopter in both eyes. Testing with 0.125% pilocarpine revealed marked constriction of the right pupil to 3 mm and no change in pupil size in the left eye, an indication of cholinergic supersensitivity in the right eye. The patient was given the diagnosis of isolated postganglionic internal ophthalmoplegia due to viral illness. A pediatric neurology consultant found no abnormalities. The patient was reexamined ophthalmologically at age 14 months without a change in findings. Nineteen months later, when he was 27 months old, his mother noted that the right eye had been turning out intermittently over the past month and that he had an apparent aversion to light with frequent closure of the right eye. Examination at that time revealed full versions but a 10 prism-diopter intermittent exotropia in primary and left gaze positions with distance and near fixation. He was orthophoric in right gaze and had a slight left face turn. There was a dilated right pupil with anisocoria greater in light than dark. Instillation of topical 0.125% pilocarpine again showed cholinergic supersensitivity of the right pupil. He was diagnosed with an intermittent right exotropia with strabismic amblyopia, possibly exacerbated by defocusing due to accommodative insufficiency accompa-nying his right internal ophthalmoplegia. He was treated with 0.125% pilocarpine in the right eye to aid accommodation and to minimize blur that would result in amblyopia and patching of the left eye 4 hours daily to reduce amblyopia. On reexamination when he was 28 months old, the exotropia had increased to 20 prism-diopters, and there was a question of slightly reduced supraduction-in-adduction of the right eye attributed to a previously unrecognized Brown syndrome. The increase in the exotropia was attributed to poor compliance. Results for the remainder of the examination were unchanged. On reexamination when he was 31 months old, the exotropia was slightly decreased and a supraduction-in- adduction deficit was not noted. At ages 32 and 35 months, examinations disclosed that visual acuity was 20/40 in each eye by Lea optotypes. Versions were again full, and an exophoriawas measured at 10 prism-diopters in distance fixation and 10 prism-diopters in near fixation. On reexamination when he was 37 months old, his mother reported increased frequency of the exotropia over the past week. Visual acuity was 20/25 in each eye. There was a 14 prism-diopter intermittent exotropia in primary position with a definite supraduction-in-adduction deficit in the right eye with resultant left hypertropia in left gaze. There was no ptosis, and results of a pupillary examination were unchanged. Cholinergic supersensitivity of the right pupil to dilute pilocarpine was again demonstrated. Neither preservation of pupil constriction to a near target nor segmental sphincter palsy was noted. Signs of aberrant reinnervation involving the pupil or lid were not detected. A pediatric neurologic examination again showed no other abnormalities. Brain and orbit MRI, obtained for the first time, showed an arachnoid cyst compressing the third cranial nerve within the right interpeduncular cistern (Fig. 1). Craniotomy showed that the arachnoid cyst was densely adherent to the third cranial nerve. Postoperatively, the patient immediately developed a complete right third cranial nerve palsy. He subsequently underwent strabismus surgery at age 5, consisting of a right medial rectus resection of 6.75 mm and a right lateral rectus recession of 8.5 mm with ½ tendon width supra-placement, producing a 20 prism-diopter exotropia in primary position at distance and near fixation. At age 7, his visual acuity was 20/50 in the right eye and 20/20 in the left eye. DISCUSSION Our patient was seen at age 8 months with isolated internal ophthalmoplegia and pupillary cholinergic super-sensitivity. At age 27 months, he developed a small intermittent exotropia and a supraduction deficit that led to the diagnosis of a third cranial nerve palsy attributed to compression by an arachnoid cyst in the interpeduncular cistern. The persistent isolated internal ophthalmoplegia accompanied by cholinergic supersensitivity had originally been misdiagnosed as a postganglionic (ciliary ganglion or ciliary nerve) lesion, presumably of postviral origin. Isolated internal ophthalmoplegia has many causes. In a patient with head trauma, biopsy demonstrated segmental tearing of fibers on the medial aspect of the third cranial nerve (10). There have also been several reports of nontraumatic persistent and isolated (or nearly isolated) internal ophthalmoplegia, with or without cholinergic supersensitivity, in otherwise healthy patients who later developed more complete third cranial nerve palsies owing to compressive lesions (5-9). Most of these patients have had intracranial aneurysms, and they often reported persistent headache. Other signs of third cranial nerve or other neurologic dysfunction usually followed. In patients with tentorial herniation, more complete third cranial nerve palsy and other signs of neurologic dysfunction, including mental status changes and hemi-paresis, have usually followed within hours (11). Wilhelm et al (5) reported a 33-year-old woman in whom isolated internal ophthalmoplegia was initially diagnosed as an Adie tonic pupil, followed 14 years later by other signs of third cranial nerve dysfunction, leading to 193 Arachnoid Cyst and Third Cranial Nerve Palsy J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 the eventual diagnosis of a neurinoma of the third cranial nerve. Their patient had asymptomatic anisocoria and was found to have light-near dissociation, vermiform iris movements, and cholinergic supersensitivity. She returned with the complaint of diplopia 14 years later. No ophthalmologic examinations occurred in the interim between presentation and diagnosis 14 years later. At that point, the affected pupil measured 9 mm in darkness, there was pupillary areflexia to light and near stimuli, and incomplete ipsilateral impairment of adduction, supra-duction, and infraduction, as well as ptosis with aberrant regeneration involving the lid. This patient differs from ours in four respects. First, the patient was an adult. Second, the patient had light-near dissociation. Third, the patient had more extensive signs of third cranial nerve palsy when the correct diagnosis was made 14 years later. The ophthalmoplegia may well have evolved over several years as its duration was uncertain. Fourth, the cause of the third cranial nerve palsy was a neurinoma, an intrinsic lesion, rather than an arachnoid cyst, an external compressive lesion, as in our patient. Werner et al (9) reported a 10-month-old infant with anisocoria and internal ophthalmoplegia as the only signs of third cranial nerve compression by a cisternal endoder-mal cyst The patient also demonstrated cholinergic supersensitivity. Light-near dissociation and segmental sphincter palsy were not reported. As in the patient of Wilhelm et al (5), Adie tonic pupil was incorrectly diagnosed. Unlike our patient, however, results of brain MRI and CT studies (not displayed in the report) were initially reported as normal. After an interval of only 6 weeks, the infant developed impaired adduction, supra-duction, and infraduction of the affected eye. Aberrant regeneration involving the lid or pupil was not described. A repeat brain MRI demonstrated a cystic mass adjacent to the cisternal portion of the third cranial nerve. The resected mass was a neurenteric cyst. Like our patient, this infant had significant residual third cranial nerve palsy requiring multiple strabismus procedures. This patient differed from ours, however, in that the third cranial nerve palsy evolved much more rapidly. The interval between presentation and correct diagnosis, based on new signs of external ophthalmoplegia, was only 6 weeks, in contrast to 19 months in our patient. That patient also differed from ours in that CT and MRI failed to demonstrate the compressive lesion at the time of presentation. Our patient's delayed diagnosis was based on the presumption that the finding of isolated internal ophthal-moplegia and cholinergic supersensitivity precluded a pre-ganglionic lesion. However, Jacobson (2) had earlier described cholinergic supersensitivity to 0.1% pilocarpine in 9 (69%) of 13 patients with preganglionic third cranial nerve palsies. The presence of supersensitivity was not related to the cause of the third cranial nerve dysfunction or FIG. 1. Brain MRI performed 29 months after original presenta-tion. A. T2 axial MRI demonstrates round high signal (arrow) in the right interpeduncular cistern com-pressing the cerebral peduncle in the course of the right third cranial nerve. B. Precontrast T1 sagittal MRI shows the cyst (arrow) in the region of the right third cranial nerve C. Precontrast T1 coronal MRI shows partial effacement of the right inter-peduncular cistern by the arachnoid cyst (arrow). D. Postcontrast T1 coronal MRI shows slight rim en-hancement of the cyst. 194 q 2008 Lippincott Williams & Wilkins J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 Ashker et al to the time between onset and testing, but it was related to the extent of associated iris sphincter palsy and to the extent of anisocoria. Unlike our patient, some of Jacobson's patients with long-standing preganglionic third cranial nerve palsies had other features usually attributed to postganglionic damage, including light-near dissociation and segmental iris sphincter palsy. He attributed the cholinergic supersensitivity to transsynaptic degeneration of postganglionic fibers in chronic preganglionic third cranial nerve disorders. In a later prospective study of several patients with congenital, ischemic, compressive, and traumatic third cranial nerve palsies, Jacobson (3) found cholinergic supersensitivity to pilocarpine with all causes except ischemia. He concluded that a large, poorly reactive pupil supersensitive to pilocarpine does not exclude a pregangli-onic lesion. In a further study comparing the degree of cholinergic supersensitivity in patients with preganglionic and postganglionic third cranial nerve palsy, Jacobson and Vierkant (4) found that the degree of cholinergic supersensitivity is similar regardless of the site of injury. Several other studies have demonstrated cholinergic supersensitivity of the iris sphincter in patients with preganglionic lesions (12-16) (Table 1). Other pupillary findings classically associated with postganglionic lesions, including light-near dissociation, segmental palsy, and a tonic response, have been documented in some patients with preganglionic third cranial nerve lesions (2,12-16). It therefore seems that all of the ‘‘classic'' pupillary signs traditionally associated with postganglionic palsy may appear, either alone or in combination, in patients with preganglionic lesions. The mechanisms producing tonicity, segmental palsy, and light-near dissociation are probably independent of cholinergic supersensitivity. Although Wirtschafter et al (17) postulated that the tonic near response in Adie pupil results from intracameral release of acetylcholine, there is no direct experimental evidence for this phenomenon. The tonic near response seems better explained by aberrant reinnervation. Segmental sphincter palsy is ascribed to sectoral innervation of the sphincter by the short posterior ciliary nerves. Supporting evidence for this mechanism is also provided by the existence of sectoral corneal hypesthesia in many patients with Adie syndrome (18). However, sectoral denervation could occur with limited damage to fascicles of the preganglionic third cranial nerve even though there is no direct evidence for such a segmental organization in the preganglionic portion of the nerve. Light-near dissociation in Adie syndrome has been attributed to aberrant reinnervation of the iris sphincter by misdirected postganglionic fibers intended for the ciliary muscle (1). This mechanism seems plausible because postganglionic axons intended for the ciliary muscle outnumber those intended for the iris sphincter by about 20:1 (19). However, aberrant reinnervation of the sphincter has also been documented in patients with compressive preganglionic third cranial nerve palsy, in whom sphincter contractions occur in synchrony with eye movements (20). The mechanism of cholinergic supersensitivity in patients with preganglionic third cranial nerve lesions is not well understood. However, Jacobson (2) argues that it is a direct consequence of sphincter denervation. First, supersensitivity is not found in patients with pupil-sparing third cranial nerve palsies, even those with compressive lesions. Second, the amount of cholinergic supersensitivity is highly correlated with the amount of anisocoria. Finally, loss of supersensitivity occurs with resolution of sphincter palsy. Several mechanisms have been proposed to explain cholinergic supersensitivity of the iris sphincter in patients with preganglionic third cranial nerve lesions. Jacobson (2) TABLE 1. ‘‘Classic'' postganglionic features in third cranial nerve palsy Cholinergic Supersensitivity Segmental Palsy Light-Near Dissociation Tonic Response Wilhelm et al (5) (1 case) 1/1 1/1 1/1 NR Werner et al (9) (1 case) 1/1 NR NR NR Jacobson (2-4) (31cases)* 11/31 NR NR* 2/31 Coppeto et al (4 cases) 4/4 NR 4/4 4/4 Slamovits et al (14) (3 cases) 3/3 0/2, 1 NR NR 0/2, 1 NR Ponsford (13) (14 cases) 14/14 NR NR NR Ford et al (16) (5 cases) NR NR 2/4, NR 1 1/1, NR 4 *Although Jacobson looked at light-near dissociation, he recorded it together with abnormal pupil reactions during ductions, not as a separate finding. NR, not reported in the paper. 195 Arachnoid Cyst and Third Cranial Nerve Palsy J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 demonstrated in normal subjects that pupils dilated with hydroxyamphetamine would constrict more than untreated pupils in response to dilute pilocarpine (2). Loewenfeld and Newsome (21) also found that in response to pilocarpine, pupils dilated with cocaine constricted more than untreated pupils. It is unlikely, however, that size alone is responsible for cholinergic supersensitivity as in the study of Jacobson (2), the amount of anisocoria was not correlated with net constriction to dilute pilocarpine. Coppeto et al (12) suggested that cholinergic supersensitivity and a tonic pupillary response in patients with third cranial nerve palsy could result from either transsynaptic degeneration followed by aberrant post-ganglionic reinnervation or from aberrant reinnervation of preganglionic fibers to the ciliary ganglion. He considered the concurrence of cholinergic supersensitivity with other classic signs of postganglionic denervation as evidence supporting a transsynaptic mechanism. However, as discussed above, other plausible preganglionic mecha-nisms may explain these features. In addition, cholinergic supersensitivity occurs too soon after the onset of third cranial nerve palsy in some patients to be explained by transsynaptic degeneration. Slamovits et al (14) and Jacobson (2) observed cholinergic supersensitivity within 5-10 days of the onset of third cranial nerve palsy in some patients. Slamovits et al (14) attributed the rapid-onset supersensitivity to damage to third cranial nerve fibers that do not synapse in the ciliary ganglion. However, there is very little anatomic evidence to support the existence of such a direct pathway. Several lines of experimental evidence suggest that postganglionic denervation is not required for the de-velopment of cholinergic supersensitivity. Supersensitivity or subsensitivity may be produced by any process that alters the state of parasympathetic stimulation of the iris sphincter. Supersensitivity may be produced in experimen-tal animals by continuous exposure to darkness (22,23) or by prolonged treatment with topical cholinergic blockers (24). Subsensitivity may be produced by continuous exposure to light (23) or prolonged treatment with acetylcholinesterase inhibitors (22,25). These studies suggest that the primary determinant of cholinergic sensitivity is the concentration of acetylcholine at the neuromuscular junction, independent of the preganglionic or postganglionic mechanism that regulates the intensity of parasympathetic stimulation. Another unusual feature of our patient was the etiology of his isolated third nerve palsy, namely an arachnoid cyst. Arachnoid cysts are an uncommon cause of third cranial nerve palsy and comprise approximately 1% of nontraumatic intracranial masses (26). These cysts consist of clear fluid enclosed in reduplicated layers of arachnoid (27). Their MRI signal characteristics are identical to those of cerebrospinal fluid (CSF). These cysts probably originate from maldevelopment of the leptomeninges in the prenatal or early postnatal period (27,28). By far the most common location for arachnoid cysts is the middle cranial fossa, where they are most often asymptomatic (27). Isolated or nearly isolated cranial neuropathies have been described in association with optic neuropathy (29) and with third (30-33), fourth (34), fifth (35,36), sixth (37,38), seventh (39), eighth (40,41), and tenth (42) cranial neuropathies. Isolated palsies of the third, fourth, and sixth cranial nerves have been reported in association with arachnoid cysts in the middle cranial fossa (37,32), Meckel's cave (35), quadrigeminal cistern (34), interpeduncular cistern (30), cavernous sinus (31), and suprasellar cistern (29). With the exception of the patient reported by McAvoy et al (38), in whom there was antecedent head trauma and increased intracranial pressure, most cases of cranial nerve palsy due to arachnoid cyst have been slowly progressive. The number of case reports is too small to permit generalizations about the outcome of ocular motor palsy due to arachnoid cysts. In addition, the cysts are quite heterogeneous in terms of location, size, and chronicity. In the only reported case of an intracavernous arachnoid cyst causing third cranial nerve palsy, no therapy was attempted (31). Of 3 patients with relatively small cysts causing ocular motor palsies, 2 had complete recovery after drainage and excision (35,30), although 1 required reoperation for a recurrence (35). The third patient, who had an acute presentation of a hemorrhagic cyst, was left with a complete third cranial nerve palsy (33). Of 2 patients with ocular motor palsies owing to very large middle fossa cysts, 1 was treated with a cystoperitoneal shunt and had a residual partial sixth cranial nerve palsy that was functionally improved after strabismus surgery (37). A second patient had a cyst fenestration for a large middle fossa cyst producing both visual loss and third cranial nerve palsy (32). Vision was unchanged after cyst fenestration despite a decrease in the size of the cyst. The postoperative status of the third cranial nerve palsy was not mentioned. In retrospect, several atypical features of our patient's history and examination might have led to earlier diagnosis of a compressive third cranial nerve palsy. When the ocular motility defect was first noted at age 27 months, he was seen with intermittent exotropia. Although versions were full, the ocular misalignment was incomitant, suggesting a medial rectus palsy. Although the patient's age and level of cooperation made precise measurements difficult and the misalignment was small, the pupillary abnormality should have suggested a partial third cranial nerve palsy and led to neuroimaging studies and earlier diagnosis. Cholinergic supersensitivity, usually considered typical of postgangli-onic lesions, should be recognized as also occurring with 196 q 2008 Lippincott Williams & Wilkins J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 Ashker et al preganglionic lesions (2-4). Moreover, our patient lacked the other typical features of postganglionic damage, namely pupillotonia, light-near dissociation, and segmental sphinc-ter palsy. Although these latter features may occasionally be present in preganglionic lesions, their absence is un-common in postganglionic lesions (1). Postganglionic lesions causing such pupillary abnormalities are distinctly unusual in infants (43,44), and diagnosis should not rest on the finding of cholinergic supersensitivity alone. Given these considerations, one should have a low threshold for obtaining neuroimaging to evaluate for a compressive lesion in children with the ocular motility and pupillary findings we have described in our patient. REFERENCES 1. Thompson HS. Adie's syndrome: some new observations. Trans Am Ophthalmol Soc 1977;75:587-626. 2. Jacobson DM. Pupillary response to dilute pilocarpine in pre-ganglionic 3rd nerve disorders. Neurology 1990;40:804-8. 3. Jacobson DM. A prospective evaluation of cholinergic supersensi-tivity of the iris sphincter in patients with oculomotor nerve palsies. Am J Ophthalmol 1994;118:377-83. 4. Jacobson DM, Vierkant RA. Comparison of cholinergic supersen-sitivity in third nerve palsy and Adie's syndrome. J Neuroophthalmol 1998;18:171-5. 5. Wilhelm H, Klier R, Toth B, et al. Oculomotor nerve paresis starting as isolated internal ophthalmoplegia. Neuroophthalmology 1995;15: 211-5. 6. Albayram S, Ozer H, Sarici A, et al. Unilateral mydriasis without ophthalmoplegia-a sign of neurovascular compression? Case report. Neurosurgery 2006;58:E582-3. 7. Payne JW, Adamkiewicz J Jr. Unilateral internal ophthalmoplegia with intracranial aneurysm. Am J Ophthalmol 1969;68:349-52. 8. Gale A, Crockard HA. Transient unilateral mydriasis with basilar aneurysm. J Neurol Neurosurg Psychiatry 1982;45:565-6. 9. Werner M, Bhatti MT, Vaishnav H, et al. Isolated anisocoria from an endodermal cyst of the third cranial nerve mimicking an Adie's tonic pupil. J Pediatric Ophthalmol Strabismus 2005;42:176-9. 10. Mariak Z, Mariak Z, Lewko J, et al. Pathogenesis of primary internal ophthalmoplegia after head injury. Eur J Ophthalmol 1995;5:56-8. 11. Plum F, Posner JB. The Diagnosis of Stupor and Coma. 3rd ed. Philadelphia: FA Davis; 1980:290-1. 12. Coppeto JR, Ribeiro Monteiro ML, Young D. Tonic pupils following oculomotor nerve palsies. Ann Ophthalmol 1985;17:585-8. 13. Ponsford JR, Bannister R, Paul EA. Methacholine pupillary responses in third nerve palsy and Adie's syndrome. Brain 1982; 105:583-97. 14. Slamovits TL, Miller NR, Burde RM. Intracranial oculomotor nerve paresis with anisocoria and pupillary parasympathetic hypersensi-tivity. Am J Ophthalmol 1987;104:401-6. 15. Behrens MM. Pupil in clinical diagnosis: failure of the light reaction. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1977;83: 827-31. 16. Ford FR, Walsh FB, King A. Clinical observations on the pupillary phenomena resulting from regeneration of the third nerve with especial reference to the Argyll Robertson pupil. Bull Johns Hopkins Hosp 1941;68:309-18. 17. Wirtschafter JD, Volk CR, Sawchuk RJ. Transaqueous diffusion of acetylcholine to denervated iris sphincter muscle: a mechanism for the tonic pupil syndrome (Adie syndrome). Ann Neurol 1978;4:1-5. 18. Purcell JJ Jr, Krachmer JH, Thompson HS. Corneal sensation in Adie's syndrome. Am J Ophthalmol 1977;84:496-500. 19. Warwick R. The ocular parasympathetic nerve supply and its mesencephalic sources. J Anat 1954;88:71-93. 20. Czarnecki JS, Thompson HS. The iris sphincter in aberrant regeneration of the third nerve. Arch Ophthalmol 1978;96:1606-10. 21. Loewenfeld IE, Newsome DA. Iris mechanics. I. Influence of pupil size on dynamics of pupillary movements. Am J Ophthalmol 1971; 71:347-62. 22. Bito LZ, Dawson MJ. The site and mechanism of the control of cholinergic sensitivity. J Pharmacol Exp Ther 1970;175:673-84. 23. Bito LZ, Dawson MJ, Petrinovic L. Cholinergic sensitivity: normal variability as a function of stimulus background. Science 1971;172: 583-5. 24. Smith EL 3rd Redburn DA, Harwerth RS, et al. Permanent alterations in muscarinic receptors and pupil size produced by chronic atropinization in kittens. Invest Ophthalmol Vis Sci 1984;25:239-43. 25. Bito LZ, Baroody RA. Gradual changes in the sensitivity of rhesus monkey eyes to miotics and the dependence of these changes on the regimen of topical cholinesterase inhibitor treatment. Invest Ophthalmol Vis Sci. 1979;18:794-801. 26. Wester K, Hugdahl K. Arachnoid cysts of the left temporal fossa: impaired preoperative cognition and postoperative improvement. J Neurol Neurosurg Psychiatry 1995 Sep;59:293-8. 27. Rengachary SS,Watanabe I. Ultrastructure and pathogenesis of intra-cranial arachnoid cysts. J Neuropathol Exp Neurol 1981;40:61-83. 28. Kumagai M, Sakai N, Yamada H, et al. Postnatal development and enlargement of primary middle cranial fossa arachnoid cyst recognized on repeat CT scans. Childs Nerv Syst 1986;2:211-5. 29. Miyamoto T, Ebisudani D, Kitamura K, et al. Surgical management of symptomatic intrasellar arachnoid cysts-two case reports. Neurol Med Chir (Tokyo) 1999;39:941-5. 30. Lesser RL, Geehr RB, Higgins DD, et al. Ocular motor paralysis and arachnoid cyst. Arch Ophthalmol 1980;98:1993-5. 31. Barr D, Kupersmith MJ, Pinto R, et al. Arachnoid cyst of the cavernous sinus resulting in third nerve palsy. J Neuroophthalmol 1999;19:249-51. 32. Callaway MP, Renowden SA, Lewis TT, et al. Middle cranial fossa arachnoid cysts: not always a benign entity. Br J Radiol 1998;71: 441-3. 33. Ide C, De Coene B, Gilliard C, et al. Hemorrhagic arachnoid cyst with third nerve paresis: CT and MR findings. AJNR Am J Neuroradiol 1997;18:1407-10. 34. Ohtsuka K, Hashimoto M, Nakamura Y. Bilateral trochlear nerve palsy with arachnoid cyst of the quadrigeminal cistern. Am J Ophthalmol 1998;125:268-70. 35. Wormer BA, Noll M, Rahim T, et al. Recurrent arachnoid cyst of Meckel's cave mimicking a brain stem ischaemia: report of a rare case. Zentralbl Neurochir 2003;64:76-9. 36. Achilli V, Danesi G, Caverni L, et al. Petrous apex arachnoid cyst: a case report and review of the literature. Acta Otorhinolaryngol Ital 2005;25:296-300. 37. Salvin JH, Repka MX, Miller MM. Arachnoid cyst resulting in sixth nerve palsy in a child. J Pediatr Ophthalmol Strabismus 2007;44: 53-4. 38. McAvoy CE, Best R, Sharkey JA, et al. Symptomatic arachnoid cyst presenting as a sixth nerve palsy. Eye 2001;15:548-50. 39. Pirotte B, Morelli D, Alessi G, et al. Facial nerve palsy in posterior fossa arachnoid cysts: report of two cases. Childs Nerv Syst 2005;21: 587-90. 40. Go¨nu¨ l E, Izci Y, Onguru O. Arachnoid cyst of the cerebellopontine angle associated with gliosis of the eighth cranial nerve. J Clin Neurosci 2007;14:700-2. 41. Falcioni M, Caruso A, Taibah A, et al. Arachnoid cysts of the petrous apex in a patient with vestibular schwannoma. Otolaryngol Head Neck Surg 2000;123:657-8. 42. Hayden MG, Tornabene SV, Nguyen A, et al. Cerebellopontine angle cyst compressing the vagus nerve: case report. Neurosurgery 2007; 60:E1150. 43. Firth AY. Adie syndrome: evidence for refractive error and accommodative asymmetry as the cause of amblyopia. Am J Ophthalmol 1999;128:118-9. 44. Brodsky MC, Baker RS, Hamed LM. Pediatric Neuro-Ophthalmol-ogy. New York: Springer-Verlag; 1996:109-10. 197 Arachnoid Cyst and Third Cranial Nerve Palsy J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 |