| OCR Text |
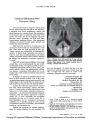
Show ORIGINAL CONTRIBUTION A Case of Acute Posterior Multifocal Placoid Pigment Epitheliopathy With Recurrent Stroke Katie Luneau, MD, Nancy J. Newman, MD, Sunil Srivastava, MD, and Valerie Biousse, MD Abstract: A 43-year-old man who had visual loss from acute posterior multifocal placoid pigment epitheliopathy (APMPPE) developed a right middle cerebral artery territory infarction a few weeks after the visual loss occurred and shortly after corticoste-roid therapy was tapered. He was then treated continuously with oral low-dose prednisone and cyclophosphamide but presented with recurrent cerebral infarction 6 months later, shortly after cyclo-phosphamide was replaced with azathioprine. Neu-rologic complications of APMPPE are exceedingly rare, with only 18 other well-documented cases of APMPPE in the English and French literature. Cerebral vasculitis was the presumed mechanism in most patients, but only 2 patients had pathologic confirmation. There have been only 3 reported cases of recurrent cerebral infarction, all occurring during corticosteroid taper. Because neurologic complica-tions of APMPPE are rare, it is reasonable to reserve neuroimaging for patients who have unusual head-aches or other new neurologic manifestations. (J Neuro-Ophthalmol 2009;29:111-118) Acute posterior multifocal placoid pigment epitheliop-athy (APMPPE) is characterized by ophthalmic mani-festations consisting of multifocal cream-colored placoid lesions at the level of the choroid (1,2). It usually affects both eyes, men and women equally, and mostly young Departments of Ophthalmology (KL, NJN, SS, VB), Neurology (NJN, VB), and Neurological Surgery (NJN), Emory University School of Medicine, Atlanta, Georgia. This study was supported in part by a department grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, NY, and by Core Grant P30-EY06360 (Department of Ophthalmology) from the National Institutes of Health, Bethesda, MD. NJN is a recipient of a Research to Prevent Blindness Lew R. Wasserman Merit Award. Address correspondence to Valerie Biousse, MD. Neuro-Ophthalmology Unit, Emory Eye Center, 1365-B Clifton Rd. N.E., Atlanta, GA 30322; E-mail: vbiouss@emory.edu adults (2). Patients typically present with isolated decreased visual acuity. The fundus lesions generally resolve spon-taneously over a few weeks, leaving alterations in the pigment epithelium. Fluorescein angiography demonstrates characteristic hypofluorescent lesions in the early phase of the angiogram ("block early") followed by hyperfluores-cence ("stain late") (1,2). The visual prognosis is usually good, often with spontaneous visual recovery without treatment. Systemic corticosteroids are sometimes used in treatment but without proven efficacy (1-26). APMPPE rarely includes cerebral vasculopathy, usually manifesting as stroke. Only 18 patients with cere-bral vasculopathy have been described in the English and French literature (2-13). We report a patient with APMPPE who had two cerebral infarctions, the first occurring within weeks of onset of the ophthalmic manifestations, shortly after corticosteroid treatment was tapered, and the second occurring 6 months later, when cyclophosphamide treat-ment was replaced with azathioprine treatment. Recurrent strokes are extraordinarily rare in APMPPE (3,6,8), and all have occurred during corticosteroid taper (2,6,8). CASE REPORT A 43-year-old white man presented with bilateral visual loss. He had hypertension and long-standing hearing loss on the left, was obese, and was a cigarette smoker. Two weeks before his visual complaints, he had a fever of 101F He subsequently developed a severe diffuse headache and was treated with antibiotics for presumed sinusitis. Soon thereafter he developed a central scotoma in his right eye, and 5 days later a similar scotoma in his left eye. Results of brain MRI were normal. The headache persisted. Visual acuity was count fingers in his right eye and 20/200 in his left eye, and there were multiple placoid cream-colored lesions in both maculas (Fig. 1). Goldmann visual fields showed central scotomas in both eyes. A retinal fluorescein angiogram showed early blockage of the macular lesions and late staining suggestive of APMPPE (Fig. 2). Results of an extensive infectious and inflammatory work-up were negative. Lumbar puncture showed 253 white cells/mm3 with 84% lymphocytes, normal glucose, and mildly elevated protein (57 mg/dL). Cytology J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 111 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Luneau et al FIG. 1. Fundus photography performed at the initial examination shows yellow-white placoid lesions in the macular region of both eyes. and culture results were negative. These findings were considered consistent with lymphocytic meningitis. In-travenous methylprednisolone (250 mg every 6 hours) was administered for 3 days, followed by a taper of oral prednisone over 10 days. Two weeks later he developed an acute left hemi-paresis. Repeat MRI with diffusion images of the brain showed acute ischemia in the right middle cerebral artery distribution (Fig. 3). MRA suggested decreased flow within the distal right middle cerebral artery branches. A catheter cerebral angiogram demonstrated mild stenosis and dilation of branches of the right middle cerebral artery and anterior cerebral artery, suggesting small vessel vasculopathy (Fig. 4), presumably from vasculitis. Intravenous methyl-prednisolone (250 mg every 6 hours) was administered for 3 days, followed by a slow taper of oral prednisone to 10 mg/day Treatment with cyclophosphamide (150 mg by mouth daily) was begun and continued for 6 months. The hemiparesis resolved over a few weeks. Six months later cyclophosphamide was discontinued and replaced by 50 mg azathioprine, and a maintenance dose of 10 mg/day prednisone. Two weeks after discontinuation of cyclophospha-mide, his headaches recurred, associated with confusion and dizziness. Brain MRI/MRA showed findings consistent with a new right middle cerebral artery (parietal) infarction. Repeat lumbar puncture showed 10 cells/mm3 with normal protein. He received 3 days of intravenous methylprednis-olone (250 mg every 6 hours), and treatment with 150 mg cyclophosphamide daily was reinstated. His symptoms improved over a few days. Three months later, his condition was stable with a regimen of 150 mg/day cyclophosphamide and 10 mg/day prednisone. The neurologic deficits continued to improve, with a lingering mild decrease in finger movement of the left hand. Visual acuity had improved to 20/80 in the right eye and 20/60 in the left eye with persistent alteration of the retinal pigment epithelium. Nine months later, he was still being treated with 150 mg/day cyclophosphamide and 5 mg/day prednisone. Results of the neuro-ophthalmologic examination were unchanged. DISCUSSION Our patient had two cerebral infarctions after his diagnosis of ophthalmic manifestations of APMPPE. The first infarction occurred within 2 weeks of the onset of the ophthalmic manifestations and shortly after corticosteroid FIG. 2. Fluorescein angiography of the right eye performed at the initial examination shows hypofluorescence of the placoid lesions in the early phase (A) and hyperfluorescence in the later phase (B), consistent with acute posterior multifocal placoid pigment epitheliopathy. (Insets indicate elapsed time in minutes and seconds from antecubital vein dye injection.) 112 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Recurrent Stroke with APMPPE J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 FIG. 3. FLAIR axial MRI shows multifocal hyperintensities in the right basal ganglia (A, arrow) and in the distal territory of the right middle cerebral artery (B, arrows), consistent with infarction. treatment was tapered. The second infarction developed 6 months later, shortly after cyclophosphamide was replaced with azathioprine, suggesting that an exacerbation of central nervous system (CNS) vasculopathy may have been related to the change in medical therapy. APMPPE rarely includes stroke. Table 1 presents a summary of the 18 well-documented reports of APMPPE associated with proven or presumed cerebral vasculitis or with cerebral venous thrombosis (2-13). Although ophthalmic APMPPE affects men and women equally, our literature review suggests that neuro-logic complications are more frequent in men (Table 1) (2-13). Among the neurologic complications of APMPPE , headaches and cerebrospinal fluid (CSF) pleocytosis are most common (2,8,26). It is unclear, however, whether these features initiate more serious complications associ-ated with cerebral vasculitis. Cerebral complications, principally stroke, are most likely to occur simultaneously or within a few weeks of the ophthalmic diagnosis. In 3 patients, they have occurred simultaneously (3,13), and in 15 patients, within a few days to 6 years (median: 3 weeks) (2-12). In 1 patient, the ophthalmic manifestations occurred 3 months after the neurologic manifestations (3) (Table 1). Only 3 cases had a recurrent cerebral infarction (3,6,8), which developed 4 days after corticosteroids were discontinued in 1 patient (6) and during corticosteroid taper 1 week and 3 months after the first stroke (3,8). CNS vasculopathy associated with APMPPE in-volves small and large arteries (lacunar and territorial infarctions). Only 10 of the reported patients had catheter cerebral angiography, with 9 showing small vessel vasculo-pathy. Lumbar puncture showed lymphocytosis in 9 of 12 patients (white blood cell count range 5-253 cells/mm3) and elevated protein in 7 of 13 patients (range 45-156 mg/dL), suggesting an inflammatory mechanism. However, the presumption of cerebral "vasculitis" as the pathologic process underlying neurologic involvement in APMPPE is mainly based on imaging findings (2-15). Multifocal segmental vessel narrowing observed on cerebral angiog-raphy is not specific to vasculitis and could also reflect vasospasm or other vasculopathies. There are only 2 case reports of patients with APMPPE that contain cerebral histopathologic descriptions (4,5). Only one report, an autopsy study, included both ocular and cerebral histo-pathologic findings (4). At the ocular level, granulomatous inflammation was seen beneath the RPE, not near the vessels, supporting inflammation but not the expected choroidal vasculitis. At the cerebral level, there was focal granulomatous vasculitis affecting large cerebral arteries in agreement with the only other documented histopathologic FIG. 4. Catheter cerebral angiography (A, lateral view; B, frontal view) shows irregularity and mild stenosis of the branches of the right middle cerebral and anterior cerebral arteries (arrows), consistent with vasculopathy. 113 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Luneau et al 114 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Recurrent Stroke with APMPPE J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 115 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Luneau et al 116 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Recurrent Stroke with APMPPE J Neuro-Ophthalmol Vol 29, No. 2, 2009 117 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Luneau el al study of cerebral vasculitis in association with APMPPE (5). One patient had vasculitis demonstrated on muscle biopsy (10). All other patients, including ours, are presumed to have had vasculitis without pathologic confirmation (27). Three of the reported patients with APMPPE had fatal cerebral infarctions (4,5,9), which occurred 3 days (4), 2 weeks (5), and 3 weeks (9) after visual loss. Most other reported strokes in patients with APMPPE produced only minor residual neurologic deficits (2,3,6,10,12). Patients with APMPPE and neurologic manifestations have gener-ally been treated with corticosteroids (16 of 19 patients), either orally (9 patients) or intravenously (7 patients). Five patients also received immunosuppressive azathioprine or cyclophosphamide (3,8,10,12). The two reported patients with APMPPE associated with cerebral venous thrombosis (3,13) had a good neurologic outcome. The small number of reported cases does not allow definite recommendations regarding the management of patients with APMPPE limited to the eyes. However, because neurologic manifestations (usually stroke) are so rare in APMPPE, it is reasonable to limit brain imaging to patients developing unusual headaches or other neurologic symptoms. Imaging should include a brain MRI with diffusion images. Treatment should be based on the severity of clinical presentation, ranging from corticosteroids alone for headaches to other immunosuppressive agents for patients with imaging evidence of cerebral vasculopathy Although only very few cases provide pathologic support for the theory that the underlying mechanism is inflam-matory, it is reasonable to prescribe high-dose infravenous corticosteroids and other immunosuppressive therapy in patients with associated cerebral vasculopathy, as recom-mended for patients with primary CNS vasculitis (8). REFERENCES 1. Gass JD. Acute posterior multifocal placoid pigment epitheliopathy. Arch Ophthalmol 1968;80:177-85. 2. Holt WS, Regan CD, Trempe C. Acute posterior multifocal placoid pigment epitheliopathy. Am J Ophthalmol 1976;81: 403-412. 3. O'Halloran HS, Berger JR, Lee WB, et al. Acute multifocal placoid pigment epitheliopathy and central nervous system involvement: nine new cases and review of the literature. Ophthalmology 2001;108: 861-8. 4. de Vries JJ, den Dunnen WF, Timmerman EA, et al. Acute posterior multifocal placoid pigment epitheliopathy with cerebral vasculitis: a multisystem granulomatous disease. Arch Ophthalmol 2006;124: 910-3. 5. Wilson CA, Choromokos EA, Sheppard R. Acute posterior multifocal placoid pigment epitheliopathy and cerebral vasculitis. Arch Ophthalmol 1988;106:796-800. 6. Smith CH, Savino PJ, Beck RW, et al. Acute posterior multifocal placoid pigment epitheliopathy and cerebral vasculitis. Arch Neurol 1983;40:48-50. 7. Sigelman J, Behrens M, Hilal S. Acute posterior multifocal placoid pigment epitheliopathy associated with cerebral vasculitis and homonymous hemianopia. Am J Ophthalmol 1979;88:919-24. 8. Comu S, Verstraeten T, Rinkoff JS, et al. Neurological manifestations of acute posterior multifocal placoid pigment epitheliopathy. Stroke 1996;27:996-1001. 9. Hammer ME, Grizzard WS, Travies D. Death associated with acute, multifocal, placoid pigment epitheliopathy. Arch Ophthalmol 1989; 107:170-1. 10. Bewermeyer H, Nelles G, Huber M, et al. Pontine infarction in acute posterior multifocal placoid pigment epitheliopathy. J Neurol 1993; 241:22-6. 11. Weinstein JM, Bresnick GH, Bell CL, et al. Acute posterior multifocal placoid pigment epitheliopathy associated with cerebral vasculitis. J Clin Neuroophthalmol 1988;8:195-201. 12. Stoll G, Reiners K, Schwartz A, et al. Acute posterior multifocal placoid pigment epitheliopathy with cerebral involvement. J Neurol Neurosurg Psychiatry 1991;54:77-9. 13. Kline DC, Vitale A, Warner JE. Acute multifocal placoid pigment epitheliopathy associated with cavernous sinus thrombosis. Ocular Immunol Inflamm 2007;15:443-6. 14. Jones NP Acute posterior multifocal placoid pigment epitheliopathy. Br J Ophthalmol 1995;79:384-9. 15. Annesley WH, Tomer TL, Shields JA. Multifocal placoid pigment epitheliopathy Am J Ophthalmol 1973;76:511-8. 16. Damato BE, Nanjiani M, Foulds WS. Acute posterior multifocal placoid pigment epitheliopathy: a follow-up study. Trans Ophthalmol Soc UK 1983;103:517-22. 17. Isashiki M, Koide H, Yamashita T, et al. Acute posterior multifocal placoid pigment epitheliopathy associated with diffuse retinal vas-culitis and late haemorrhagic macular detachment. Br J Ophthalmol 1986;70:255-9. 18. Kirkham TH, Ffytche TJ, Sanders MD. Placoid pigment epitheliop-athy with retinal vasculitis and papillitis. Br J Ophthalmol 1972;56: 875-80. 19. Bird AC, Hamilton AM. Placoid pigment epitheliopathy presenting with bilateral serous retinal detachment. Br J Ophthalmol 1972;56:881-6. 20. Van Buskirk EM, Lessell S, Friedman E. Pigmentary epitheliopathy and erythema nodosum. Arch Ophthalmol 1971;85:369-72. 21. Deutman AF, Oosterhuis JA, Boen-tan TN, et al. Acute posterior multifocal placoid pigment epitheliopathy. Pigment epitheliopathy of choriocapillaritis? Br J Ophthalmol 1972;56:863-74. 22. Jacklin HN. Acute posterior multifocal placoid pigment epitheliop-athy and thyroiditis. Arch Ophthalmol 1977;95:995-7. 23. Laatikainen LT, Immonen IJ. Acute posterior multifocal placoid pigment epitheliopathy in connection with acute nephritis. Retina 1988;8:122-4. 24. Dick DJ, Newman PK, Richardson J, et al. Acute posterior multifocal placoid pigment epitheliopathy and sarcoidosis. Br J Ophthalmol 1988;72:74-7. 25. Kersten DH, Lessell S, Carlow TJ. Acute posterior multifocal placoid pigment epitheliopathy and late-onset meningo-encephalitis. Ophthalmology 1987;94:393-6. 26. Bullock JD, Fletcher RL. Cerebrospinal fluid abnormalities in acute posterior multifocal placoid pigment epitheliopathy. Am J Oph-thalmol 1977;84:45-9. 27. Bousser MG, Biousse V Small vessel vasculopathies affecting the central nervous system. J Neuroophthalmol 2004;24:56-61. 118 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. |