| OCR Text |
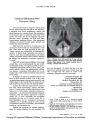
Show ORIGINAL CONTRIBUTION Combined Functional MRI and Diffusion Tensor Imaging Analysis of Visual Motion Pathways Linda J. Lanyon, PhD, Deborah Giaschi, PhD, Simon Au Young, MSc, Kevin Fitzpatrick, BSc, Lu Diao, Bruce H. Bjornson, MD, and Jason J. S. Barton, MD, PhD Background: Motion perception may be preserved after damage to striate cortex (primary visual cortex, area V1). Awareness and normal discrimination of fast-moving stimuli have been observed even in the complete absence of V1. These facts suggest that motion-sensitive cortex (the V5/MT complex or V5/ MT+) may be activated by direct thalamic or collicular inputs that bypass V1. Such projections have been identified previously in monkeys but have not been shown in humans using neuroimaging techniques. Methods: We used diffusion tensor imaging (DTI) tractography to visualize white matter fiber tracts connecting with V5/MT+ in 10 healthy volunteers. V5/MT+ was localized for each subject using functional MRI (fMRI). Functional activity maps were overlaid on high-resolution anatomical images and registered with the diffusion-weighted images to define V5/MT+ as the region of interest (ROI) for DTI tractography analysis. Fibers connecting to V1 were excluded from the analysis. Results: Using conservative tractography parame-ters, we found connections between the V5/MT+ region and the posterior thalamus and/or superior colliculus in 4 of 10 subjects. Conclusions: Connections between the V5/MT+ region and the posterior thalamus and/or superior colliculus may explain visual motion awareness in the absence of a functioning V1. (J Neuro-Ophthalmol 2009;29:96-103) Some patients rendered cortically blind by lesions to the striate cortex may nevertheless have some residual visual abilities. Patients with ‘‘blindsight'' (1) deny conscious awareness of stimuli in their scotomatous visual fields, yet are able to make perceptual discriminations or perform associated visuomotor control at better than chance levels (2-5). Several reports suggest that striate cortex (area V1, primary visual cortex) activity is necessary for conscious visual perception (6-10). In its absence, how-ever, either some degraded conscious vision or blindsight may remain, particularly if structures such as the superior colliculus, lateral geniculate nucleus (LGN), and extras-triate cortex are not directly lesioned. Preservation of conscious perception seems to be particularly likely in relation to visual motion (2,11-18). Although there are many cortical regions with motion sensitivity, key areas are the middle temporal area (MT), medial superior temporal cortex (MST), and the fundus of the superior temporal cortex (FST), known collectively in humans as the V5/MT complex or V5/MT+ (19). In the macaque, many medial temporal neurons remain visually responsive and sensitive to motion direction after striate cortex lesions or cooling (20). Human and animal studies suggest several candidate pathways that project to V5/MT+ and bypass V1 (21,22). V5/MT+ could receive input directly from the LGN or from the superior colliculus via the pulvinar nucleus of the thalamus. Studies in monkeys have found direct projections to V5/MT+ from the pulvinar nucleus (23-26) and the LGN (27). However, whether similar pathways exist in humans is not known. Human Vision and Eye Movement Laboratory (LJL, LD, JJSB), Departments of Medicine (Neurology) (LJL, LD, JJSB), Ophthalmology and Visual Sciences (LJL, DG, LD, JJSB), Psychology (LJL, LD, JJSB), and Pediatrics (Neurology) (BHB), University of British Columbia, Vancouver, British Columbia, Canada; and Children's Brain Mapping Centre, BC Children's Hospital, Vancouver, British Columbia, Canada (DG, SAY, KF, BHB). Initial results of this study were presented at the Society for Neuroscience meeting, November 2007, San Diego, CA. L.L. was supported by a Postdoctoral Fellowship from the Michael Smith Foundation for Health Research. D.G. was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada. J.B. was supported by a Senior Scholar Award from the Michael Smith Foundation for Health Research and a Canada Research Chair. Address correspondence to Linda Lanyon, PhD, Human Vision and Eye Movement Laboratory, Room 365, 3rd Floor Research Labs, VGH Eye Care Centre, 2550 Willow Street, Vancouver, BC, Canada V5Z 3N9; E-mail: llanyon@eyecarecentre.org 96 J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Visual Motion Pathways J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 In this study, we used neuroimaging techniques to investigate the presence of subcortical connections to V5/MT+ in healthy human subjects. We first used functional magnetic resonance imaging (fMRI) to localize V5/MT+ in each subject because the exact anatomical location of this area is subject to individual variation (28,29). Diffusion tensor imaging (DTI) tractography was then used to image potential fiber tracts emanating from the V5/MT+ region of interest (ROI) that did not involve V1. This combined fMRI/DTI analysis allowed us to determine whether there were any tracts traveling between V5/MT+ and the thalamus and midbrain. METHODS Subjects Ten healthy volunteers (4 men and 6 women, aged 20-37 years) participated in this experiment. The protocol was approved by the institutional review boards of Vancouver General Hospital and the University of British Columbia, and all subjects gave informed consent in accordance with the Declaration of Helsinki. Apparatus and Procedure Scans were conducted on a Phillips 3-T magnetic resonance scanner at the University of British Columbia High Field MRI Centre. The scanning session consisted of a T1 high-resolution structural scan (magnetization prepared rapid acquisition gradient echo [MPRAGE] with sensitivity encoding [SENSE]; time to recovery [TR] ~10 msec, time to echo [TE] 6 msec, field of view 212 X 212 mm, slice thickness 1.1 mm, 256 X 256 reconstruction matrix, voxel size 1.1 X 1.1 mm, and reconstructed voxel size 0.83 X 0.83mm), a T2* fMRI scan using echo planar imaging (SENSE; TR 2000 ms, TE 30 msec, field of view 240 X 240 mm, 36 interleaved axial slices of 3-mm thickness with 1-mm gap, 80 X 80 matrix, 128 X 128 reconstruction matrix, voxel size 3 X 3 mm, and reconstructed voxel size 1.88 X 1.88 mm), and three consecutive diffusion-weighted scans using 32 diffusion sensitizing directions (SENSE; TR ranging from 5586 to 6307 msec, TE 69 msec, B value 700 s/mm2, field of view 212 X 212 mm, 56-60 axial slices of 2.2-mm thickness, no gap, 96 X 96 matrix, 256 X 256 reconstructed matrix, voxel size 2.21 X 2.21 mm, and reconstructed voxel size 0.83 X 0.83 mm). The purpose of the functional scan was to localize the region of the cortex sensitive to motion for each subject because there are large individual differences in the exact location of this region (28,29). Subjects fixated the center of a display containing white dots, each of 20-minute size (0.3°, 5 X 5 pixels), at 2% density on a black background. The dots moved radially in and out from the center of the pattern for 14 seconds at a speed of 7.5 per second and then remained stationary for 14 seconds. This process was repeated for six cycles. DTI and fMRI Analysis We used DTI tractography techniques to probe white matter tract topology ((30); for reviews see (31,32)). Although DTI studies commonly refer to ‘‘white matter'' topology, unmyelinated axonal membranes also lead to anisotropic diffusion (33). However, myelin is thought to modulate the degree of anisotropy (34). For each individual subject, we used DTI Studio version 2.40 software (Hiang and Mori, http://lbam.med.jhmi.edu/dtiuser/dtiuser.asp) to perform streamlined fiber tracking, based on the fiber assignment by the continuous tracking method (FACT) (35) for the whole brain. The gradient directions used during the scan were corrected for individual subjects' head posi-tions using program code provided by the Kennedy Krieger Institute (http://godzilla.kennedykrieger.org/~jfarrell/ software_web.htm), recommended for use with DTI Studio. Whole brain tractography was performed such that fiber tracking was started at voxels with a fractional anisotropy (FA) value greater than 0.2 and ended when a voxel was encountered with an FA value less than 0.2. A threshold of 0.2 is commonly used for this type of deterministic tractography (for example, (36)) because FA values less than 0.2 tend to be found in grey matter, whereas values FIG. 1. Regions of interest (ROIs) of V5/MT+ based on functional activity (arrows). As is normal for visual motion tasks, functional activity is also present in posterior occipital cortex (arrowheads) because activity in this region is above the threshold used to identify V5/MT+ activity. The posterior occipital activity is not used for DTI analysis. L, left; R, right. 97 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Lanyon et al greater than 0.2 are found in white matter (37). The maximum turning angle (the angle over which neighboring tensor principal vectors are joined to form a tract) was set to 45. Both of these parameters are in the middle of the range recommended for DTI Studio. To confirm the reliability of the whole brain tracto-graphy in each subject, we identified the optic radiation and corpus callosum white matter tracts resulting from the tractography. Functional activity was analyzed using Brain Voyager QX versions 1.7 and 1.8 (Brain Innovation, www.brainvoyager. com). Activity in the moving versus the stationary dot conditions was compared using a general linear model, and areas of peak activity for motion were determined using a threshold of q < 0.001. Only regions containing a cluster of at least 50 consecutive significantly activated voxels were accepted. The functional data were overlaid on the high-resolution anatomic images (obtained from the T1 structural scans), and the resulting images were registered with the diffusion images for analysis in DTI Studio. In DTI Studio, the ROI for tractography was drawn manually on the basis of the location of V5/MT+ functional activity (Fig. 1). V5/MT+ is located very close to fibers of the optic radiation, and, therefore, our ROI drawing was particularly conservative in that region. The posterior occipital region was specifically excluded from the ROI using the drawing tool ‘‘NOT'' function so that fibers traveling to V1 were removed from analysis. Fibers connected to the V5/MT+ ROI were then analyzed by visual inspection to determine the number that connected with the posterior thalamus and/or superior colliculus, which were identified based on anatomical landmarks from a detailed MRI atlas (38). For larger fiber bundles, the statistics feature of DTI Studio was used to count fibers when individual fibers could not be determined visually. As a control, we performed a second analysis in which we drew an ROI deep in the intraparietal sulcus (IPS). Our analysis was performed at an individual level because the functional localizer, on which tractography was based, gave the specific location of V5/MT+ for each subject. The fiber analysis provided information about FIG. 2. All fibers tracked from V5/MT+ for Case 3. The slice views focus on the pulvinar nucleus. The top left panel shows a rotation-enabled three-dimensional view of the fibers against a semitransparent two-dimensional axial slice. The top right panel shows an axial slice view, and the bottom row shows sagittal and coronal slice views. Each slice view contains only those fibers present in that particular slice, with fibers being randomly colored for visualization purposes. V5/MT+ functional activation was not present in the left hemisphere for this subject. From the right hemisphere V5/MT+, several fibers were tracked to the pulvinar. 98 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Visual Motion Pathways J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 fibers traveling to and from this location in each individual and revealed individual variation in results. RESULTS We found direct connections between V5/MT+ and subcortical locations in 4 of our 10 subjects (Table 1; Figs. 2 through 5). All 4 of these subjects (Cases 1-4) had fibers connecting V5/MT+ with the pulvinar nucleus. Two sub-jects (Cases 1 and 4) had fibers connecting with the superior colliculus. One subject (Case 4) had fibers connecting with the region of the LGN. An example of the tractography results in Case 6, typical of the remaining subjects, is shown in Fig. 6. We do not believe the difference in tractography results is due to an age-related effect in the diffusion data because the 4 subjects in whom connections were found included the oldest subject (age 37) and 2 of the youngest subjects (age 22). From the control analysis, none of the subjects had fibers extending from our IPS ROI to the thalamus, superior colliculus, or LGN. Fibers to the right pulvinar nucleus were numerous in Case 3. This could be because visual motion-related acti-vation was unilateral (it was bilateral in our other subjects), and this pathway might be preferentially strengthened in this hemisphere in this individual. However, any quanti-tative comparison of DTI reconstructive tracts is made with caution because the results relate to lines propagated through the tensor map rather than to numbers of real fibers. In 3 of these 4 subjects, we also found fibers that extended inferiorly in the brainstem through the pons and a small number that proceeded into the medulla. Feedback connections from V5/MT+ to the pons are known to exist in monkeys (23,39). A few of the pontine fibers continued to be tracked through the middle cerebellar peduncle to the cerebellum, but the extensively crossing fibers present in the pons increase the likelihood of tractography artefacts in this case. FIG. 3. Fibers tracked from V5/MT+ for Case 3 to the medial pulvinar nucleus only. Fibers are shown in 3 dimensions against a two-dimensional axial slice. To establish whether this individual variation in our results was due to differences in the size of the region of functional activation, we performed a further control set of analyses. We identified the ROI for tractography using a large oval around but extending beyond the region of functional activity in the subjects for whom we did not find fibers to the thalamus. This analysis revealed fibers to the thalamus (pulvinar) in only one additional subject. We performed a further set of tractography analyses for this subject using ROIs systematically extending in size in different directions. These analyses revealed that the TABLE 1. Summary of subjects in whom fibers were tracked from V5/MT+ to specific non-cortical regions* Case Age (y) Superior Colliculus Pulvinar LGN Cerebellum or Cerebellar Peduncles Brain Stem 123 4 22 37 22 25 400 6 23 46 19 000 4 + + 108 ++, large bundle of fibers. *The number of reconstructed tracts is shown. Note that a quantitative analysis is not appropriate in relation to DTI reconstructed tracts. Numbers of fibers relate to the lines of tractography that have propagated through the tensor map and do not express the number of real fiber tracts. 99 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Lanyon et al FIG. 4. All fibers tracked from V5/MT+ for Case 4. Tractography in this subject revealed numerous V5/MT+ fibers connecting with the superior colliculus, pulvinar nucleus, and LGN, as well as the pons and cerebellum. The slice views focus on the fibers tracked to the vicinity of the LGN in the right hemisphere. This region is highlighted with a black oval on the axial and coronal views. pulvinar fibers resulted from extending the ROI medially far beyond the functionally defined region into the neighboring white matter of the optic radiation in one particular slice. Extending the ROI in other directions and by lesser amounts did not produce fibers. Therefore, we consider this result to be due to fibers of the optic radiation from V1 and conclude that this subject, like the other remaining 5 subjects, does not have fibers tracking directly between V5/MT+ and the thalamus. DISCUSSION Our study used fMRI to identify the V5/MT+ regions in each subject and then used DTI to visualize fiber tracts projecting between V5/MT+ (but not V1) and other structures, in particular the posterior thalamus and superior colliculus. Given the proximity of the optic radiations to V5/MT+, we identified the V5/MT+ region conservatively, as well as excluding any fibers that projected to posterior occipital cortex. With this conservative approach, our DTI data suggested the presence of tracts linking V5/MT+ with the pulvinar nucleus and the LGN in the posterior thalamus and the superior colliculus in 4 of 10 subjects. A recent DTI investigation (40) also showed connections between the pulvinar and the V5/MT+ region by tracking fibers from the pulvinar as the ROI, but V5/MT+ was not localized by fMRI in that study. It has been suggested that a direct connection to V5/MT+ from the superior colliculus, pulvinar nucleus, or LGN could mediate residual visual motion perception in the absence of V1 (22). In monkeys, MST and FST are reciprocally connected to the pulvinar and project to the pons (23). Feedback connections have been found from MT to the pulvinar nucleus, LGN, superior colliculus, and pons in the macaque monkey (39) and the prosimian primate Galago senegalensis (41). More relevant for the issue of residual vision and blindsight are feed-forward thalamic and collicular projections to V5/MT+ in monkeys that have also been described (21-27). In squirrel (24) and rhesus (26) monkeys, the pulvinar projects to the MT. The MT is the major cortical target of the medial nucleus of the inferior pulvinar in owl monkeys (25). 100 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Visual Motion Pathways J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 FIG. 5. Fibers tracked for Case 4 in the right hemisphere between V5/MT+ and LGN only. Fibers are shown in 3 dimensions against a two-dimensional axial slice. We found fibers connecting V5/MT+ to the vicinity of the superior colliculus in 2 of our 10 subjects. Lesions of the superior colliculus alone have little effect on medial temporal responses in macaque monkeys, but combining collicular lesions with V1 lesions renders the MT visually unresponsive (42). This information suggests that the superior colliculus may be a key source of input in the absence of V1, either through direct projections to extra-striate cortex or via a relay in the pulvinar. Another DTI study (43) reported extensive projections between the superior colliculus and visual cortical areas in two hemispherectomized patients with residual motion percep-tion but limited projections in hemispherectomized patients with no conscious awareness of vision. Our results confirm a similar variability in the projections between V5/MT+ and subcortical regions in normal subjects. It should be noted that the technical limitations of DTI tractography can produce some variability, particularly when tracking through regions of complex fiber structure. However, the diversity in our results could also reflect a true intersubject variability of pathways, as suggested by others (43). Variability in the viability of projections from subcortical structures to V5/MT+ may explain why only some patients with V1 FIG. 6. All fibers tracked from V5/MT1 for Case 6. No fibers were tracked to the thalamus or midbrain in this subject. Similar results were found in Cases 5 and 7-10. Fibers in the left hemisphere extend into the temporal lobe (fibers are shown in three-dimensional view against an axial slice view at the level of the thalamus). lesions have residual motion perception or blindsight. Large group studies suggest that only a minority of patients with cortical visual loss have residual abilities (44-46). Although there are many potential factors that could contribute to this variability, such as the extent of training, age at onset (2,47-49), and degree of additional damage to the extrastriate cortex (2,47-51), variations in the pre-morbid anatomy of pathways between the superior colliculus/pulvinar nucleus and the V5/MT+ region may be another important factor. The fact that we found such pathways in a minority of healthy subjects is consistent with the finding that only a minority of patients with cortical visual loss have blindsight. We stress that our DTI analyses cannot provide defini-tive proof of these projections, inasmuch as the technique cannot tell us whether these projections are from sub-cortical structures to V5/MT+, from V5/MT+ to subcortical structures, or both. The data from monkey studies suggest that there are feed-forward and feedback pathways con-necting these structures, but DTI cannot provide data on the direction of information flow. Rather, these results can only be viewed as consistent with suggestions that, if visual information can be transmitted directly from subcortical 101 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Lanyon et al regions to V5/MT+ without mediation by V1, such tracts should exist. Further work showing whether the presence of these tracts correlates with residual visual perception would be an important step in verifying the role of these tracts in vision. Acknowledgments We thank our subjects for their time in participating in the experiment. We also extend grateful thanks to Natashka Pollock and Nailyn Rasool for participating in parts of the tractography analysis, and to George Malcolm and Robert Orlando, who participated in early stages of the project. Thanks go to staff, especially Trudy Harris, at the University of British Columbia High Field MRI Centre, and to Burkhard Maedler of Phillips Medical Systems for his helpful advice. REFERENCES 1. Weiskrantz L. Consciousness and commentaries. In: Hameroff SR, Kaszniak AW, Scott AC, eds. Towards a Science of Consciousness Ii-the Second Tucson Discussions and Debates. Cambridge, MA: M.I.T. Press; 1998:371-7. 2. Blythe IM, Kennard C, Ruddock KH. Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain 1987;110: 887-905. 3. Corbetta M, Marzi CA, Tassinari G, et al. Effectiveness of different task paradigms in revealing blindsight. Brain 1990;113:603-16. 4. Perenin MT, Jeannerod M. Residual vision in cortically blind hemifields. Neuropsychologia 1975;13:1-7. 5. Perenin MT, Ruel J, He´caen H. Residual visual capacities in a case of cortical blindness. Cortex 1980;16:605-12. 6. Celesia GG, Bushnell D, Toleikis SC, et al. Cortical blindness and residual vision: is the ‘‘second'' visual system in humans capable of more than rudimentary visual perception? Neurology 1991;41:862-9. 7. Cowey A, Stoerig P. Visual detection in monkeys with blindsight. Neuropsychologia 1997;35:929-39. 8. Goebel R, Muckli L, Zanella FE, et al. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res 2001;41:1459-74. 9. Merigan WH, Nealey TA, Maunsell JH. Visual effects of lesions of cortical area V2 in macaques. J Neurosci 1993;13:3180-91. 10. Stoerig P. Varieties of vision: from blind responses to conscious recognition. Trends Neurosci 1996;19:401-6. 11. Riddoch G. Dissociation of visual perceptions due to occipital injuries, with especial reference to appreciation of movement. Brain 1917;40:15-57. 12. Barbur JL, Watson JD, Frackowiak RS, et al. Conscious visual perception without V1. Brain 1993;116:1293-302. 13. Giaschi D, Jan JE, Bjornson B, et al. Conscious visual abilities in a patient with early bilateral occipital damage. Dev Med Child Neurol 2003;45:772-81. 14. Morland AB, Jones SR, Finlay AL, et al. Visual perception of motion, luminance and colour in a human hemianope. Brain 1999; 122:1183-98. 15. Mestre DR, Brouchon M, Ceccaldi M, et al. Perception of optical flow in cortical blindness: a case report. Neuropsychologia 1992;30: 783-95. 16. Perenin MT. Discrimination of motion direction in perimetrically blind fields. Neuroreport 1991;2:397-400. 17. Ptito A, Lepore F, Ptito M, et al. Target detection and movement discrimination in the blind field of hemespherectomized patients. Brain 1991;114:497-512. 18. Schoenfeld MA, Heinze HJ, Woldorff MG. Unmasking motion-processing activity in human brain area V5/MT+ mediated by pathways that bypass primary visual cortex. Neuroimage 2002;17: 769-79. 19. Zeki S, Watson JD, Lueck CJ, et al. A direct demonstration of functional specialization in human visual cortex. J Neurosci 1991;11: 641-9. 20. Rodman HR, Gross CG, Allbright TD. Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal. J Neurosci 1989;9:2033-50. 21. Cowey A, Stoerig P. The neurobiology of blindsight. Trends Neurosci 1991;14:140-5. 22. Stoerig P, Cowey A. Blindsight in man and monkey. Brain 1997;120: 535-59. 23. Boussaoud D, Desimone R, Ungerleider LG. Subcortical connections of visual areas MST and FST in macaques. Vis Neurosci 1992;9: 291-302. 24. Cusick CG, Scripter JL, Darensbourg JG, et al. Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. J Comp Neurol 1993;336:1-30. 25. Lin CS, Kaas JH. Projections from the medial nucleus of the inferior pulvinar complex to the middle temporal area of the visual cortex. Neuroscience 1980;5:2219-28. 26. Rockland KS, Andresen J, Cowie RJ. Single axon analysis of pulvinocortical connections to several visual areas in the macaque. J Comp Neurol 1999;406:221-50. 27. Sincich LC, Park KF, Wohlgemuth MJ, et al. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 2004;10:1123-8. 28. Dumoulin SO, Bittar RG, Kabani NJ, et al. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex 2000;10: 454-63. 29. Watson JD, Myers R, Frackowiak RS, et al. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 1993; 3(2): 79-94. 30. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103: 247-54. 31. Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis-a technical review. NMR Biomed 2002;15: 456-67. 32. Mori S, van Zijl PC. Fiber tracking: principles and strategies-a technical review. NMR Biomed 2002;15:468-80. 33. Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med 1994;31:394-400. 34. Beaulieu C. The basis of anisotropic water diffusion in the nervous system-a technical review. NMR Biomed 2002;15: 435-55. 35. Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265-69. 36. Thomas C, Avidan G, Humphreys K, et al. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci 2009;12:29-31. 37. Cercignani M, Bozzali M, Iannucci G, et al. Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;70:311-7. 38. Duvernoy HM. The Human Brain: Surface. Three-Dimensional Sectional Anatomy with MRI, and Blood Supply, 2nd ed. New York: Springer-Verlag Wien; 1999. 39. Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 1983;3:2563-86. 40. Leh SE, Chakravarty MM, Ptito A. The connectivity of the human pulvinar: A diffusion tensor imaging tractography study. Int J Biomed Imaging 2009; doi: 10.1155/2008/789539. 102 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Visual Motion Pathways J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 41. Wall JT, Symonds LL, Kaas JH. Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in galagos. J Comp Neurol 1982;211:193-214. 42. Rodman HR, Gross CG, Allbright TD. Afferent basis of visual response properties in area MTof the macaque. II. Effects of superior colliculus removal. J Neurosci 1990;10:1154-64. 43. Leh SE, Johansen-Berg H, Ptito A. Unconscious vision: new insights into the neuronal correlate of blindsight using diffusion tractography. Brain 2006;129:1822-32. 44. Barton JJ, Sharpe JA. Smooth pursuit and saccades to moving targets in blind hemifields: a comparison of medial occipital, lateral occipital and optic radiation lesions. Brain 1997;120: 681-99. 45. Kasten E, Wuest S, Sabel BA. Residual vision in transition zones in patients with cerebral blindness. J Clin Exp Neuropsychol 1998;20: 581-98. 46. Scharli H, Harman AM, Hogben JH. Blindsight in subjects with homonymous visual field defects. J Cogn Neurosci 1999;11: 52-66. 47. Moore T, Rodman HR, Repp AB, et al. Greater residual vision in monkeys after striate cortex damage in infancy. J Neurophysiol 1996; 76:3928-33. 48. Payne BR, Lomber SG, Macneil MA, et al. Evidence for greater sight in blindsight following damage of primary visual cortex early in life. Neuropsychologia 1996;34:741-74. 49. Perenin MT, Jeannerod M. Visual function within the hemianopic field following early cerebral hemidecortication in man-I. Spatial localization. Neuropsychologia 1978;16:1-13. 50. Weiskrantz L. Residual vision in a scotoma: a follow-up study of ‘‘form'' discrimination. Brain 1987;110:77-92. 51. Weiskrantz L. Outlooks for blindsight: explicit methodologies for implicit processes. Proc R Soc Lond B Biol Sci 1990;239: 247-78. 103 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. |