| OCR Text |
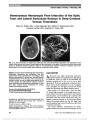
Show Cortical Vision Loss as a Prominent Feature of H1N1 Encephalopathy John H. Pula, MD, Ahmad Issawi, MD, Jeffrey R. DeSanto, MD, Jorge C. Kattah, MD Abstract: A 20-year-old woman infected with the 2009 H1N1 strain of influenza A developed bilateral visual loss. Brain MRI showed restricted diffusion of the parietal and occipital lobes, and her spinal fluid did not contain inflammatory cells. This report describes an unusual case of H1N1 influenza A virus infection primarily affecting the posterior visual pathways. Journal of Neuro-Ophthalmology 2012;32:48-50 doi: 10.1097/WNO.0b013e31823fd913 © 2012 by North American Neuro-Ophthalmology Society CASE REPORT A20-year-old woman with Type I diabetes mellitus reported 1 week of malaise and fatigue. On initial examination, her blood pressure was 106/66 mm Hg; pulse rate, 114 beats per minute; temperature, 101.9°F; respira-tions, 51 per minute; and pulse oximeter oxygen satura-tion, 92%. Arterial blood gas PO2 was 68 mm Hg (normal, 75-90 mm Hg); PCO2, 24 mm Hg (normal, 35-45 mm Hg); glucose, 193 mg/dL (normal, 70-99 mg/dL); and creatinine, 2.82 mg/dL (normal, 0.6-1 mg/dL). Chest X-ray showed bilateral patchy infiltrates. Real-time poly-merase chain reaction (PCR) sampled from pharyngeal mucosa tested positive for H1N1 influenza virus. The patient was admitted to hospital and given intra-venous fluids, piperacillin/tazobactam, azithromycin, and oseltamivir. Because of respiratory distress, she was intubated, sedated with midazolam and fentanyl, and required an oscillator for 7 days, with traditional ventila-tion for an additional 10 days. Her creatinine normalized. Blood cultures showed no growth. She developed a hospital-acquired fungal urinary tract infection treated with flu-conazole but remained normotensive and hemodynamically stable. On Hospital Day 17, she was extubated and stated that she could not see. On neurologic examination, the patient was mildly agitated and irritable. Visual acuity was hand motions in each eye. External, pupillary, and funduscopic examinations were normal. The following day, brain MRI demonstrated signal abnormalities involving the posterior parietal and occipital cortex bilaterally (Fig. 1). There was mild patchy enhance-ment in regions of signal abnormality without evidence of hemorrhage on gradient echo, nor evidence of cerebral venous thrombosis on T1-weighted imaging. Electroen-cephalography showed periodic lateralizing epileptiform discharges from the right hemisphere. Cerebrospinal fluid (CSF) analysis showed no white or red blood cells, glucose was 78 mg/dL (normal, 40-70 mg/dL), and protein was 110 mg/dL (normal, 12-60 mg/dL). CSF HSV PCR, cryptococcal screen, Gram stain, and bacterial culture were negative. On Hospital Day 20, the patient became drowsy and agitated and required reintubation for worsening respiratory function. One week later, she was extubated, and on the following day, she was alert with visual acuity of J5 in both eyes with normal pupillary testing and funduscopy. Brain MRI with FLAIR sequences showed signal abnormalities in the occipital lobes bilaterally (Fig. 2). The patient had no further respiratory decompensation but maintained a persistently flat affect. Neuropsychologic examination showed moderately reduced cognitive func-tion. Simple auditory attention was preserved. However, on tasks requiring visual attention and speed, her performance was moderately to severely impaired. Abstract visual construction was also decreased. Departments of Neurology and Neuro-ophthalmology (JHP, JCK), Neurosurgery (AI), and Radiology (JRD), University of Illinois College of Medicine at Peoria, Peoria, Illinois. The authors report no conflicts of interest. Address correspondence to John H. Pula, MD, Department of Neu-rology and Neuro-ophthalmology, University of Illinois College of Medicine at Peoria, 530 NE Glen Oak Avenue, Peoria, IL 61637; E-mail: jpula1@uic.edu 48 Pula et al: J Neuro-Ophthalmol 2012; 32: 48-50 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. DISCUSSION There are 3 types of influenza virus: A, B, and C. Influenza A is an enveloped RNA virus of multiple strains and is named for which subtypes of hemagglutinin and neuro-minidase antigen are on its surface. These subtypes include H1N1, H1N2, H3N1, H3N2, and H2N3 (1). Episodi-cally, influenza A H1N1 strains have caused outbreaks of human disease. For example, the 1918 Spanish flu epidemic implicated in encephalitis lethargica was triggered by an H1N1 strain (1). In 2009, a genetically distinct strain of H1N1 influenza A became pandemic. This strain clinically manifested mainly with respiratory problems, especially in young people. Neurologic complications of the 2009 H1N1 strain occurred primarily in children, resulting in seizures and encephalop-athy (2,3). Focal neurologic problems such as hemisensory loss caused from H1N1 were less commonly reported (4,5). Despite neurologic involvement, CSF analysis in these patients was often normal (6-9). In our patient, the elevated protein but otherwise unremarkable CSF is consistent with the CSF profile from other H1N1 encephalopathy patients (6,10). Neuroimaging abnormalities from influenza A enceph-alitis may be due to several mechanisms. Hypoxic or anoxic damage as a result of respiratory dysfunction can lead to CNS ischemia or infarction (10). Alternatively, CNS dam-age may be due to a direct infectious or parainfectious pro-cess related to the H1N1 virus. Mainly described in children, these parainfectious syndromes range from acute demyelinating encephalomyelitis to more hyperacute states, such as acute hemorrhagic leukoencephalopathy (4). Probably related, acute necrotizing encephalopathy is a syndrome mainly described in East Asian children, resulting in seizures, fever, and coma (11). Brain imaging typically demonstrates bilateral symmetric lesions of the thalami, white matter, and brainstem (7,12). In such a case, FIG. 1. Axial DWI images (A) with apparent diffusion coefficient maps (B) show signal changes in the parietal and occipital cortex bilaterally consistent with restricted diffusion. FIG. 2. Axial FLAIR images show increased signal in the occipital lobes bilaterally. Pula et al: J Neuro-Ophthalmol 2012; 32: 48-50 49 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. autopsy findings showed vasculopathy and necrotizing lesions of white matter, basal ganglia, thalami, and brain-stem (13). Neuroimaging abnormalities associated with the pan-demic 2009 H1N1 strain have been reported infrequently, usually in children (4,10,14,15). Not all neurologic symp-toms occurring in H1N1 patients produce MRI changes (5). In a series of 8 children with H1N1 encephalopathy, only 3 showed bilateral lesions of the thalami and white matter on MRI (16). Lyon et al (17) described the case of a 12-year-old girl with H1N1 encephalopathy who devel-oped seizures and lethargy and had bilateral thalamic and brainstem MRI lesions. Haktanir (15) reported a 3-year-old girl with bilateral thalamic and brainstem T2 hyperinten-sities, which showed restricted diffusion on diffusion-weighted imaging (DWI). Occipital lobe involvement due to H1N1 has been documented in 2 previously reported cases, but the young age of both patients precluded accurate assessment of visual function (18,19). The neuroimaging abnormalities in our patient raised the possibility of posterior reversible encephalopathy syn-drome. Yet the lack of clinical improvement and cytotoxic edema found on DWI are both inconsistent with this diagnosis. Hypoxia also could result in restricted diffusion but would produce imaging findings of cortical laminar necrosis, which were not present in our patient. Finally, cerebral ischemia was considered, but the vascular territories involved are not representative of hypotension or arterial thromboembolism, and there was no evidence of venous thrombosis. Thus, the pattern of symmetrical MRI abnor-malities with restricted diffusion in our case most likely represents a parainfectious encephalopathy. REFERENCES 1. Morens DM, Taubenberger JK, Fauci AS. The persistent legacy of the 1918 influenza virus. N Engl J Med. 2010;361:225-229. 2. Brock E, Varghese A, Miller L. Neurologic complications associated with novel influenza A (H1N1) virus infection in children: Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:773-778. 3. Sejvar JJ, Uyeki TM. Neurologic complications of 2009 influenza A (H1N1). Neurology. 2010;74:1020-1021. 4. Wang J, Duan S, Zhao J, Zhang L. Acute disseminated encephalomyelitis associated with influenza A H1N1 infection. Neurol Sci. 2011;32:907-909. 5. Tan K, Prema A, Leo YS. Surveillance of H1N1-related neurological complications. Lancet Neurol. 2010;9:142-143. 6. Al-Baghli F, Al-Ateeqi W. Encephalitis-associated pandemic A (H1N1) 2009 in a Kuwaiti girl. Med Princ Pract. 2011;20:191-195. 7. Fujimoto Y, Shibata M, Tsuyuki M, Okada M, Tsuzuki K. Influenza A virus encephalopathy with symmetrical thalamic lesions. Eur J Pediatr. 2000;159:319-321. 8. Morishima T, Togashi T, Yokota S, Okuno Y, Miyazaki C, Tashiro M, Okabe N. Collaborative Study Group on Influenza- Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512-517. 9. Gadoth A, Aizenstein O, Mosek A. Influenza A/H1N1 encephalitis. Neurology. 2010;75:666-667. 10. Fugate J, Lam E, Rabinstein A, Wijdicks E. Acute hemorrhagic leukoencephalitis and hypoxic brain injury associated with H1N1 influenza. Arch Neurol. 2010;67:756-758. 11. Ormitti F, Ventura E, Summa A, Picetti E, Crisi G. Acute necrotizing encephalopathy in a child during the 2009 influenza A(H1N1) pandemia: MR imaging in diagnosis and follow-up. AJNR Am J Neuroradiol. 2010;31:396-400. 12. Protheroe SM, Mellor DH. Imaging in influenza A encephalitis. Arch Dis Child. 1991;66:702-705. 13. Ng WF, Chiu SC, Lam DSY, Wong YC, Tam S, Kwong NS, Loo KT, Yuen KY. A 7-year-old boy dying of acute encephalopathy. Brain Pathol. 2010;20:261-264. 14. Chen Y, Lo C, Chang T. Novel influenza A (H1N1)-associated encephalopathy/encephalitis with severe neurological sequelae and unique image features-a case report. J Neurol Sci. 2010;298:110-113. 15. Haktanir A. MR imaging in novel influenza A(H1N1)- associated meningoencephalitis. Am J Neuroradiol. 2010;31: 394-395. 16. Zhao C, Gan Y, Sun J. Radiographic study of severe influenza-A (H1N1) disease in children. Eur J Radiol. 2011;79:447-451. 17. Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol. 2010;40:200-205. 18. Sandoval-Gutierrez JL, Arcos M, Alva L, Volkow P, Ferrari T, Quinones F, Vazquez-Perez J, Ormsby CE, Hanssen F, Resendiz A, Alcantar P, Perez Padilla R, Bautista E. Letter to the editor. Ann Neurol. 2011;69:217-218. 19. Mariotti P, Iorio R, Frisullo G, Plantone D, Colantonio R, Tartaglione T, Batocchi AP, Valentini P. Acute necrotizing encephalopathy during novel influenza A (H1N1) virus infection. Ann Neurol. 2010;68:111-114. 50 Pula et al: J Neuro-Ophthalmol 2012; 32: 48-50 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |