| OCR Text |
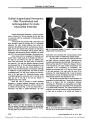
Show PHOTO ESSAY Cytomegalovirus Infection with MRI Signal Abnormalities Affecting the Optic Nerves, Optic Chiasm, and Optic Tracts Suzann Pershing, MD, Jeffrey Dunn, MD, Ahmir Khan, MD, and Yaping Joyce Liao, MD, PhD Abstract: A 49- year- old woman who had been immunosuppressed after a renal transplant developed bilateral severe visual loss. Visual acuities were finger counting and hand movements in the two eyes. Both optic nerves were pale. There were no other ophthalmic abnormalities. Brain MRI disclosed marked signal abnormalities involving the optic nerves, optic chiasm, and optic tracts. Cerebrospinal fluid polymerase chain reaction ( PCR) was posi-tive for cytomegalovirus. Treatment did not restore vision. Such extensive clinical and imaging involve-ment of the anterior visual pathway, which has been previously reported with other herpes viruses, illustrates the propensity for this family of viruses to track along axons. ( J Neuro- Ophthalmol 2009; 29: 223- 226) A 49- year- old woman who had been given an immu-nosuppressive regimen ( tacrolimus, mycophenolate, and prednisone) since renal transplantation in 2001 for IgA nephropathy, presented with a 3- month history of decreased appetite, weight loss, and bilateral visual loss. She had experienced rapidly deteriorating visual loss, which was accompanied by bilateral eye pain aggravated by eye movement, over the preceding week. Brain and spinal MRI demonstrated prominent enhancement of the optic nerves, optic chiasm, and optic FIG. 1 Postcontrast axial ( A- C) and coronal ( D- F) MRI images show intense enhancement ( arrows) of the optic nerves ( A, D), optic chiasm ( B, E), and optic tracts ( C, F) in a patient with cytomegalovirus infection. Departments of Ophthalmology ( SP, YJL) and Neurology ( JD, AK), Stanford University School of Medicine, Stanford, California. Y. J. L is supported by the Career Award in Biomedical Sciences from the Burroughs Wellcome Foundation. Address correspondence to Y. Joyce Liao, MD, PhD, Department of Ophthalmology, Stanford University Medical Center, 900 Blake- Wilbur Drive, Room W3002, Stanford, CA 94305- 5353; E- mail: yjliao@ stanford. edu J Neuro- Ophthalmol, Vol. 29, No. 3, 2009 223 tracts ( Fig. 1). On T2 sequences, these tissues showed abnormally high signal, as did some thoracic spinal cord segments. Visual acuity was counting fingers in the right eye and hand movements in the left eye. Both pupils constricted poorly to light, and there was a small left relative afferent pupillary defect. Ophthalmoscopy disclosed prominent bilateral optic nerve pallor and blurred disc margins from swelling and gliosis ( Fig. 2). There were no retinal lesions, vitreous inflammation, or anterior segment abnormalities. Lumbar puncture showed an opening pressure of 31 cm H2O with 9 white blood cells ( 70% lymphocytes) and normal protein, glucose, IgG/ albumin ratio, culture, and cytology. There were no oligoclonal bands. Spinal fluid was positive for cytomegalovirus ( CMV) by DNA polymerase chain reaction ( PCR). Serum PCR and IgG were positive for CMV, but IgM and shell vial culture were negative. Serum tests were negative for coccidiomycosis ( comple-ment fixation), cryptococcal antigen ( latex agglutination), Borrelia burgdorferi ( IgG and IgM), JC virus ( DNA PCR), human T- lymphocyte virus ( HTLV) 1 and 2 ( IgG), herpes simplex virus ( HSV) 1 and 2 ( DNA PCR), Epstein- Barr virus ( EBV) ( DNA PCR), adenovirus ( DNA PCR), enterovirus ( DNA PCR), toxoplasma ( PCR), and West Nile virus ( IgM and IgG). Blood PCR testing was negative for JC virus, HTLV- 1 and 2, EBV, human herpes virus 6, varicella zoster virus, and adenovirus. Serologic testing for other infections showed no evidence of syphilis ( rapid plasma reagin [ RPR] and treponemal antibody by enzyme-linked immunosorbent assay [ ELISA]), HIV- 1 or 2 ( antibody), or toxoplasma ( IgG). There was no evidence of inflammatory disease, with negative antinuclear anti-bodies ( ANAs), anti- neutrophil cytoplasmic antibodies ( ANCAs) antibody panel ( including anti- myeloperoxi-dase), anti- double- stranded DNA, antiphospholipid anti-body, and neuromyelitis optica ( NMO) IgG. A total body positron emission tomography ( PET)- CT scan revealed abnormal [ 18 F] fluorodeoxyglucose uptake from the brainstem to L1, corresponding to lower extremity weakness and a T4 sensory level that had been disclosed on an interval neurologic examination. There was also intense PET activity in the distal small bowel and proximal colon, accounting for watery diarrhea. Upper gastrointestinal endoscopy revealed nonbleed-ing erosions in the gastric antrum, biopsy of which was positive for CMV ( Fig. 3), which confirmed the positive serum (< 600 copies/ mL) and spinal fluid CMV PCR analysis. Treatment with 2.5 mg/ kg ganciclovir intravenously daily led to resolution of diarrhea and electrolyte imbalance. Repeat virology studies showed negative serum and cerebrospinal fluid ( CSF) CMV by PCR and, as before, negative serum CMV IgM, positive CMV IgG, and negative CMV shell vial culture. Despite this clinical and laboratory improvement, repeat neuro- ophthalmologic evaluation at 1 month and again 2 months later showed no change in visual acuity or ophthalmic findings. Our patient developed extensive clinical and imaging involvement of both optic nerves, the optic chiasm, and the optic tracts, presumably from CMV infection. This finding illustrates the propensity for the herpes family of viruses to track along axons. There are reported cases of patients with AIDS who had CMV infection in whom MRI showed hyperintensities in both optic tracts and lateral geniculate nuclei on T2 images ( 1,2) or gadolinium enhancement along the optic nerve ( 2- 10), optic chiasm ( 2), or optic tract ( 2). Such visual pathway involvement occurs in association with progressive outer retinal necrosis ( 1,2), acute retinal necrosis ( 2), or meningitis ( 1). Varicella zoster infection in patients with AIDS has also been associated with optic nerve ( 4- 8) or optic chiasm ( 9) involvement, sometimes in the setting of FIG. 2. Optic disc pallor, gliosis from prior disc edema, and periarterial cuffing are evident in both fundi. 224 q 2009 Lippincott Williams & Wilkins J Neuro- Ophthalmol, Vol. 29, No. 3, 2009 Pershing et al progressive outer retinal necrosis ( 4). To the best of our knowledge, this is the first reported case of a patient with prominent bilateral optic nerve, optic chiasm, and optic tract involvement in CMV infection. Intracranial visual pathway involvement is typically accompanied by ocular involvement ( 1- 3). In fact, the most common ophthalmic manifestation of CMV is retinitis, followed by optic neuritis ( 11). CMV optic neuritis is currently estimated to occur in only about 4% of patients with CMV retinitis ( 11), a substantial decline from levels of approximately 30% in the years before the availability of ganciclovir ( 12). This predisposition to retina and optic nerve involvement may be due to the neurotropic nature of the virus ( 12). Iritis, keratitis, retinal vasculitis, and vein occlusion are much less common and are usually in immunocompromised patients ( 13- 17). In the era preceding active antiretroviral therapy ( HAART), CMV ocular infection in AIDS patients was associated with decreased survival, amounting to an average of 4- 7 months after diagnosis ( 11,12). With the advent of HAART therapy, CMV retinitis rates have declined substantially, from an incidence of approximately 30% pre- HAART to a 7% incidence currently ( 18). Curiously, the incidence of CMV ocular infection appears to be much lower in patients who are immunosup-pressed from causes other than AIDS, perhaps owing to more careful monitoring and prophylaxis of opportunistic infections. In one series of 12,653 published liver transplant patients, only 0.1% developed CMV retinitis ( 13). Similarly, although systemic CMV reactivation was recently described as occurring in up to 20%- 30% of critically ill patients hospitalized for more than 14 days ( 19), few appeared to develop symptomatic infection of the visual pathways. Of the methods used for diagnosis of CMV infection, PCR is most sensitive. PCR assays are able to detect CMV DNA from blood even before the onset of clinical symptoms. In contrast, conventional cell culture and shell vial assays have low sensitivity and low reproducibility ( 14- 16). Whereas recent case reports of CMV ocular infection ( 17,20) were confirmed by CMV serum and/ or aqueous PCR, older reports of CMV retinitis ( 21,22,23) were based solely on serum antibody titers consistent with acute infection. The agents used for treatment of CMV ocular infection currently include valganciclovir ( 20), ganciclovir ( 17,24), and foscarnet ( 22,24). Corticosteroids may be used in concert with antiviral therapy ( 18) to combat inflammation. Untreated CMV optic neuritis leads to substantial visual loss ( 25- 28). Timely treatment with valganciclovir has been reported to improve the prognosis ( 29). However, in one case series of 31 patients with CMV optic neuritis, the prognosis remained poor despite treatment. Vision improved in only 2 patients, was unchanged in 17, and worsened in 12 ( 30). Because CMVophthalmic infections may relapse after apparently successful treatment, affected patients should be monitored ( 12). FIG. 3. Gastric antrum biopsy shows cytomegalovirus inclusion bodies ( arrows) in low- power ( A) and high- power ( B, C) views. ( Hematoxylin and eosin stain). 225 MRI in Cytomegalovirus Infection J Neuro- Ophthalmol, Vol. 29, No. 3, 2009 REFERENCES 1. Park KH, Bang JH, Park WB, et al. Retrobulbar optic neuritis and meningoencephalitis following progressive outer retinal necrosis due to CMV in a patient with AIDS. Infection 2008; 36: 475- 9. 2. Bert RJ, Samawareerwa R, Melhem ER. CNS MR CT findings associated with a clinical presentation of herpetic acute retinal necrosis and herpetic retrobulbar optic neuritis: five HIV- infected and one non- infected patients. AJNR Am J Neuroradiol 2004; 25: 1722- 9. 3. Tornerup NR, Fomsgaard A, Nielsen NV. HSV- 1- induced acute retinal necrosis syndrome presenting with severe inflammatory orbitopathy, proptosis, and optic nerve involvement. Ophthalmology 2000; 107: 397- 01. 4. Franco- Paredes C, Bellehemeur T, Merchant A, et al. Aseptic meningitis and optic neuritis preceding varicella- zoster progressive outer retinal necrosis in a patient with AIDS. AIDS 2002; 16: 1045- 9. 5. Meenken C, van den Horn GJ, de Smet MD, et al. Optic neuritis heralding varicella zoster virus retinitis in a patient with acquired immunodeficiency syndrome. Ann Neurol 1998; 43: 534- 6. 6. Shayegani A, Odel JG, Kazim M, et al. Varicella- zoster virus retrobulbar optic neuritis in a patient with human immunodeficiency virus. Am J Ophthalmol 1996; 122: 586- 8. 7. Lee MS, Cooney EL, Stoessel KM, et al. Varicella zoster virus retrobulbar optic neuritis preceding retinitis in patients with acquired immune deficiency syndrome. Ophthalmology 1998; 105: 467- 71. 8. Friedlander SM, Rahhal FM, Ericson L, et al. Optic neuropathy preceding acute retinal necrosis in acquired immunodeficiency syndrome. Arch Ophthalmol 1996; 114: 1481- 5. 9. Greven CM, Singh T, Stanton CA, et al. Optic chiasm, optic nerve, and retinal involvement secondary to varicella- zoster virus. Arch Ophthalmol 2001; 119: 608- 10. 10. Tran C, Du Pasquier RA, Cavassini M, et al. Neuromyelitis optica following CMV primo- infection. J Intern Med. 2007; 261: 500- 3. 11. Patel SS, Rutzen RA, Marx JL, et al. Cytomegalovirus papillitis in patients with acquired immune deficiency syndrome. Visual prognosis of patients treated with ganciclovir and/ or foscarnet. Ophthalmology 1996; 103: 1476- 82. 12. Mansour AM. Cytomegalovirus optic neuritis. Curr Opin Oph-thalmol 1997; 8: 55- 8. 13. Egli A, Bergamin O, Mullhaupt B, et al. Cytomegalovirus- associated chorioretinitis after liver transplantation: case report and reviewof the literature. Transplant Infect Dis 2008; 10: 27- 43. 14. Pancholi P, Wu F, Della- Latta P. Rapid detection of cytomegalovirus infection in transplant patients. Expert Rev Mol Diagn 2004; 4: 231- 42. 15. Yoshida A. Laboratory diagnosis of cytomegalovirus infections and disease ( in Japanese). Rinsho Byori 2008; 56: 1034- 42. 16. Minjolle S, Arvieux C, Gautier AL, et al. Detection of herpesvirus genomes by polymerase chain reaction in cerebrospinal fluid and clinical findings. J Clin Virol 2002; 25: S59- S70. 17. De Silva SR, Chohan G, Jones D, et al. Cytomegalovirus papillitis in an immunocompetent patient. J Neuroophthalmol 2008; 28: 126- 37. 18. Kedhar SR, Jabs DA. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Herpes 2007; 14: 66- 71. 19. Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008; 300: 413- 22. 20. Stewart MW, Bolling JP, Mendez JC. Cytomegalovirus retinitis in an immunocompetent patient. Arch Ophthalmol 2005; 123: 572- 4. 21. Chawla HB, Ford MJ, Munro JF, et al. Ocular involvement in cytomegalovirus infection in a previously healthy adult. Br Med J 1976; 2: 281- 92. 22. Baglivo E, Leuenberger PM, Krause KH. Presumed bilateral cytomegalovirus- induced optic neuropathy in an immunocompetent person. A case report. J Neuroophthalmol 1996; 16: 14- 7. 23. England AC 3rd, Miller SA, Maki DG. Ocular findings of acute cytomegalovirus infection in an immunologically competent adult. N Engl J Med 1982; 307: 94- 5. 24. Eid AJ, Bakri SJ, Kijpittayarit S, et al. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transplant Infect Dis 2008; 10: 13- 8 25. Mansour AM, Uchida T, Rodenko G, et al. Ocular- systemic interrelationships in acquired immunodeficiency syndrome. Int J STD AIDS 1991; 2: 25- 9. 26. Neuwirth J, Gutman I, Hofeldt AJ, et al. Cytomegalovirus retinitis in a young homosexual male with acquired immunodeficiency. Ophthalmology 1982; 89: 805- 8. 27. Rosecan LR, Stahl- Bayliss CM, Kalman CM, et al. Antiviral therapy for cytomegalovirus retinitis in AIDS with dihydroxy propoxymethyl guanine. Am J Ophthalmol 1986; 101: 405- 18. 28. Winward KE, Hamed LM, Glaser JS. The spectrum of optic nerve disease in human immunodeficiency virus infection. Am J Ophthalmol 1989; 107: 373- 80. 29. Ioannidis AS, Bacon J, Frith P. Juxtapapillary cytomegalovirus retinitis with optic neuritis. J Neuroophthalmol 2008; 28: 128- 30. 30. Mansour AM, Li HK. Cytomegalovirus optic neuritis: characteristics, therapy and survival. Ophthalmologica 1995; 209: 260- 66. 226 q 2009 Lippincott Williams & Wilkins J Neuro- Ophthalmol, Vol. 29, No. 3, 2009 Pershing et al |