| Description |
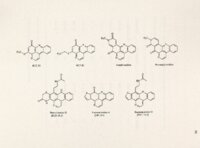
Polycyclic aromatic alkaloids derived from marine tunicates and sponges have provided chemicals that are cytotoxic in vitro against various tumor cell lines and effective in mouse tumor xenograft models. Ascididemin is a marine, pentacyclic alkaloid derived from a Didemnum sp. tunicate found off the coasts of Okinawa and New Zealand. It has potent cytotoxicity against tumor cells in vitro and in vivo. Preclinical screening at NCI revealed the in vivo antineoplastic activity of ascididemin and a semisynthetic analog BCMH -1-48. BC-l 09-1, a second semisynthetic analog, is set to enter screening shortly. Previously, ascididemin had been .reported to inhibit topo II and induce topo 11- mediated DNA cleavage. This study, however, focuses on the unique ability of ascididemin, 109 and 48 to cleave DNA even in the absence of topo. A whole plasmid assay revealed the concentration-dependency of their ability to cleave DNA and identified DIT as the sole requirement for maximal activity. Based on the shared structural features between the three analogs, a double N bay region and iminoquinone heterocyclic ring, two possible mechanisms of action were initially hypothesized: metal binding to the common phenanthroline bay region to facilitate redox cycling and generate reactive oxygen species (ROS) and direct reduction of the quinone moiety to produce ROS. The proposed Fenton-mediated mechanism of in vitro DNA cleavage was not experimentally supported. However, antioxidants catalase and reduced GSH protected against DNA cleavage in vitro and protected cultured CRO cells from toxicity, confirming the role of ROS. Therefore, we hypothesize that ascididemin and two semisynthetic analogs, 109 and 48, produce their in vitro cytotoxic effects at least in part by cleaving DNA through ROS production. The present study describes the role of ROS in ascididemin and analog-induced DNA cleavage. Anoxic conditions inhibited DNA cleavage by all three drugs. Cyclic voltammetry revealed less energy was necessary to reduce the three DNA cleaving analogs compared to two inactive ones. EPR confirmed the presence of free radicals in vitro. Treatment of mutant CRO cells identified a predilection of these drugs to induce ss breaks in cells, consistent with ROS activity. Induction of oxidative stress proteins, R0-1 and GST, was also observed in treated cells. Finally, at toxic concentrations in culture, all three drugs modulated cell accumulation in the S phase of the cell cycle, again consistent with DNA damage. It is therefore concluded that this ROS-mediated mechanism is likely responsible for ascididemin, 48 and 109 DNA cleavage and anticancer activity. |