| OCR Text |
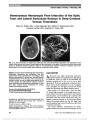
Show Anatomic Correlates of Visual Field Loss: Some Settled, Some Not Christian J. Lueck, PhD, FRCP(UK), FRACP Every reader of this Journal knows that a detailed knowledge of anatomy is fundamental to clinical neuro-ophthalmology. The retinotopic organization of the visual pathways provides an example of how an accurate diagnosis relies on a precise understanding of the relevant neural structures. In this issue, 3 articles touch on the neuroanatomy of visual field loss. Two PhotoEssays report highly unusual causes of pathology affecting the visual pathways while an original contribution looks at cytoarchitectonic probabilistic mapping, a new technique which may prove useful in the ongoing quest to define the retinotopic organisation of the primary visual cortex (V1). Liu et al (1) present the case of a young girl with Wyburn-Mason syndrome. At 6 years of age, the patient was noted to have decreased vision in her right eye associated with a retinal arteriovenous malformation (AVM). Automated perimetry at 11 years of age revealed a defect in the upper temporal visual field of her normal-appearing left eye, and subsequent imaging of her brain showed that her chiasm was compressed by a large AVM. Intracranial AVMs are occasionally documented to interfere with the retrochiasmal visual pathways in Wyburn-Mason syndrome and there have been a few reports of monocular temporal hemifield defects due to chiasmal involvement. However, a junctional scotoma from chiasmal involvement is extremely unusual. A second patient reported by Grabe et al (2) is a 20-year-old man who developed a very severe illness due to cerebral venous sinus thrombosis while camping at high altitude in Peru. Cerebral venous sinus thrombosis in this situation typically affects superficial veins, such as the superior sagittal sinus, but this patient's thrombosis involved deep cerebral veins and resulted in infarction of both thalami and surrounding brainstem structures. Following recovery several months later, a residual right homony-mous hemianopia was associated with damage to the left optic tract and lateral geniculate nucleus, both of which were clearly demonstrable on MRI. Homonymous hemianopia due to thrombosis of deep cerebral veins is rare. Though the pathology was unusual in both these cases, it is reassuring that the pattern of visual field loss was entirely consistent with the anatomic sites of the lesions. We have a very clear understanding of the anatomy of visual field loss generated by lesions located between eye and occipital cortex. However, when it comes to understanding the detailed retinotopic arrangement of the primary visual cortex (V1), things are less certain. This is an ongoing area of debate, and the article by Papageorgiou et al (3) offers an interesting new contribution. It is just over 100 years since Inouye (4) reported his observations on soldiers wounded in the Japanese wars of 1900 and 1904 to 1905. Both his studies and the subsequent work of Holmes (5,6) largely relied on correlating patterns of visual field loss with an estimate of occipital lobe damage derived from the location and superficial appearance of their patients' gunshot wounds. Apart from Inouye's erroneous inclusion of 5° of ipsilateral macular representation, both authors generated conceptually similar retinotopic maps of V1, but that published by Holmes (6) in 1945 is much more widely known. During the past 35 years, there have been dramatic advances in neuroimaging. New techniques have been applied to the question of the retinotopic organization of V1. Early studies of computed tomography (7,8) and positron emission tomography (9) yielded results compatible with the Holmes map, but the spatial resolution of both techniques was low and did not permit examination of the retinotopic map in any detail. Department of Neurology, The Canberra Hospital and Australian National University Medical School, Canberra, Australia. The author reports no conflicts of interest. Address correspondence to Christian J. Lueck, PhD, FRCP(UK), FRACP, Department of Neurology, The Canberra Hospital and Australian National University Medical School, Canberra, Australia; E-mail: christian.lueck@act.gov.au 2 Lueck: J Neuro-Ophthalmol 2012; 32: 2-4 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. The accuracy of the Holmes map was challenged when MRI became available and offered significantly higher spatial resolution. Horton and Hoyt (10) suggested that human V1 was similar to that of old world primates because they found that the representation of the central 30° of vision occupied approximately 80% of V1 compared with previous estimates by Inouye (4) and Holmes (6) of approx-imately 40% (Table 1). McFadzean et al (11) studied a larger number of patients using both CT and MRI and reported findings that supported Horton and Hoyt (10). A third MRI study by Wong and Sharpe (12) was not so consistent. These authors offered a slightly different map, this time with a somewhat smaller degree of central field magnification. Emerging techniques continue to be used in an attempt to settle this debate. Several authors have used functional MRI (13-16), and their findings appear to correlate well with Horton and Hoyt's map. More recently, 7-T MRI (17) and magnetoencephalography (18) have been applied, but precise retinotopic details from these techniques are not yet available. One factor that undoubtedly contributes to the variation in the findings of the above studies is that no 2 brains are alike. This is hardly surprising. Our faces are not structurally identical, nor are our internal organs, and the differences do not lie just in simple geometric transformations-resizing and rescaling our faces to one standard template would not render us all identical. It therefore seems rather unlikely that resizing and rescaling brains (these transformations gen-erally form an integral part of image analysis) will yield brains whose component areas (such as V1) are identically arranged. Recent studies have attempted to quantify the extent of intersubject variation by detailed examination of postmortem human brains. One study looked at the precise location of Brodmann cytoarchitectonic areas (19), whereas another study used a combination of diffusion tensor imag-ing and histology (20). Both studies demonstrated a surpris-ingly large amount of intersubject variability and both produced "probabilistic maps" that offer estimates of the likely spatial locations of given anatomical structures such as Brodmann area 17 (area V1). In fact, it appears that intersubject variability is sufficiently large that only a very small proportion of the area of cortex defined structurally as area V1 in one subject is likely to overlap spatially with the area of cortex defined as area V1 in every other subject. This degree of intersubject variability must have a major impact on any study that tries to generate a "universal template." In this issue of the Journal, Papageorgiou et al (3) have used these "probabilistic maps" to study the retinotopic organization of V1 in 2 patients who had cortical lesions responsible for small areas of peripheral visual field loss. They correlated the location of visual field loss with the precise location of the cortical lesions on MRI and concluded that their findings were more supportive of the original Holmes map (5) than either of the more recent maps suggested by MRI studies (10,12). Comparison with probabilistic maps suggested that one patient's lesion probably involved part of the optic radiation and V1 while the other's was more likely to have been centered on V1. This demonstrates how difficult it is to know exactly which functional area is being affected by a given lesion in an individual patient. Despite all the recent advances in neuroimaging, the bottom line is that the appearance of a lesion on an MRI scan still cannot tell us precisely which part of an individual patient's primary visual cortex has been damaged. To do this we will need an imaging technique that can define the unique anatomic boundaries of V1 in each patient. The debate over the retinotopic organisation of V1 seems set to continue for some time. REFERENCES 1. Liu A, Chen Y-W, Chang S, Liao YJ. Junctional visual field loss in a case of Wyburn-Mason syndrome. J Neuroophthalmol. 2011;32:42-44. 2. Grabe HM, Bapuraj JR, Wesolowski JR, Parmar H, Trobe JD. Homonymous hemianopia from infarction of the optic tract and lateral geniculate nucleus in deep cerebral venous thrombosis. J Neuroophthalmol. 2011;32:38-41. 3. Papageorgiou E, Ticini LF, Schiefer U. Peripheral homonymous hemianopia: correlation between lesion location and visual field defects by means of cytoarchitectonic probabilistic maps. J Neuroophthalmol. 2011;32:5-12. TABLE 1. Retinotopic maps of visual field projection to area V1 Author Date Methodology Number of Subjects Approximate Percentage of Cortex Devoted to Visual Field Central 15° Central 30° Inouye (4) 1909 Missile injuries 30 29* 45* Holmes and Lister (5) Holmes (6) 1919 1945 Missile injuries 23 (+) 23* 43* Horton and Hoyt (10) 1991 MRI 3 68* 83† Wong and Sharpe (12) 1999 MRI 14 37† 68* Dumoulin and Wandell (15) Wandell and Winawer (16) 2008 2011 Functional MRI 6 53* 77* *Approximate value measured from text figures. †Stated in text. Lueck: J Neuro-Ophthalmol 2012; 32: 2-4 3 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 4. Inouye T. Die Sehstörungen bei Schussverletzungen der kortikalen Sehsphäre nach Beobachtungen an Versundeten der letzten Japanische Kriege. Leipzig, Germany: W. Engelmann, 1909. 5. Holmes G, Lister WT. Disturbances of vision from cerebral lesions with special reference to the cortical representation of the macula. Brain. 1916;39:34-73. 6. Holmes G. Ferrier lecture: the organization of the visual cortex in man. Proc R Soc Lond B Biol Sci. 1945;132:348-361. 7. McAuley DL, Ross Russell RW. Correlation of CAT scan and visual field defects in vascular lesions of the posterior visual pathways. J Neurol Neurosurg Psychiatry. 1979;42:298-311. 8. Spector RH, Glaser JS, David NJ, Vining DQ. Occipital lobe infarctions: perimetry and computed tomography. Neurology. 1981;31:1098-1106. 9. Fox PT, Miezin FM, Allman JM, van Essen DC, Raichle ME. Retinotopic organisation of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987;7:913-922. 10. Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991;109:816-824. 11. McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Br J Ophthalmol. 1994;78:185-190. 12. Wong AMF, Sharpe JA. Representation of the visual field in the human occipital cortex. A magnetic resonance imaging and perimetric correlation. Arch Ophthalmol. 1999;117:208-217. 13. Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181-192. 14. Vanni S, Henriksson L, James AC. Multifocal fMRI mapping of visual cortical areas. Neuroimage. 2005;27:95-105. 15. Dumoulin SO, Wandell BA. Population receptive field estimates in human visual cortex. Neuroimage. 2008;39:647-660. 16. Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Res. 2011;51:718-737. 17. Sánchez-Panchuelo RM, Francis ST, Schluppeck D, Bowtell RW. Correspondence of human visual areas identified using functional and anatomical MRI in vivo at 7 T. [published online ahead of print September 30, 2011]. J Magn Reson Imaging. 18. Perry G, Adjamian P, Thai NJ, Holliday IE, Hillebrand A, Barnes GR. Retinotopic mapping of the primary visual cortex- a challenge for MEG imaging of the human cortex. Eur J Neurosci. 2011;34:652-661. 19. Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotactic space-where and how variable? Neuroimage. 2000;11: 66-84. 20. Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach J, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092-1105. 4 Lueck: J Neuro-Ophthalmol 2012; 32: 2-4 Editorial Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |