| OCR Text |
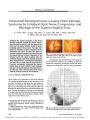
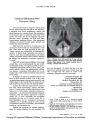
Show ORIGINAL CONTRIBUTION Bilateral Mesial Occipital Lobe Infarction After Cardiogenic Hypotension Induced by Electrical Shock Roheena Kamyar, MD and Jonathan D. Trobe, MD Abstract: A 28-year-old man developed cerebral blindness from infarction of both mesial occipital lobes after cardiogenic hypotension induced by electrical shock. He remained globally encephalo-pathic for several weeks, but his most enduring deficit was bilateral homonymous hemianopias with mac-ular sparing. Cerebral visual loss after electrical injury has been sparsely reported. It has been attributed to direct thermal injury of the skull or posterior dural venous sinuses. We suggest that cerebral blindness after cardiogenic hypotension in which there is no thermal injury to the scalp be attributed to hypotensive infarction of the mesial occipital lobes, which lie in the terminal domain of the posterior cerebral arteries. (J Neuro-Ophthalmol 2009;29:107-110) Cerebral visual loss after electrical injury has been sparsely reported (1-4). It has been attributed to local thermal injury to the brain parenchyma (1) or dural sinuses (2). In one report that involved cardiac arrest (3), reversible binocular visual loss was attributed to vasogenic edema caused by the electrical injury. In one case of persistent homonymous hemianopia after a mild electrical shock to the left hand (4), the mechanism remained uncertain. We report a patient who suffered severe binocular visual and memory loss after electrical shock, causing ventricular tachycardia and systemic hypotension. Brain imaging was consistent with visual cortex infarction, which we attribute to the hypotension. Department of Ophthalmology and Visual Sciences (RK, JDT), Kellogg Eye Center (RK, JDT), University of Michigan, Ann Arbor, Michigan; and Department of Neurology (JDT), University of Michigan Medical Center, Ann Arbor, Michigan. Address correspondence to Jonathan D. Trobe, MD, Kellogg Eye Center, 1000 Wall Street, Ann Arbor, MI 48109; E-mail: jdtrobe@ umich.edu CASE REPORT A 28-year-old male roofer sustained an electrical injury of 220 Valternating current (AC) while moving a large extension ladder with a coworker. He was on the ground securing the ladder on which his coworker was perched. The ladder pitched backwards and came into contact with over-head power lines. Conducted through the ladder, the electri-cal discharge immediately caused his coworker to be thrown to the ground. Our patient continued to grip the ladder for approximately 15 seconds before falling to the ground. According to witnesses, he displayed tonic-clonic movements for several minutes and then ‘‘turned blue.'' Bystander resuscitation began promptly and continued for approximately 6 minutes until emergency medical techni-cians arrived. At that point, cardiac monitors were positioned and the patient was found to be in pulseless ventricular tachycardia. He received three AC cardiac shock treatments and two rounds of epinephrine and atropine before he developed sinus rhythm. He was estimated to have been in cardiac arrest for approximately 10 minutes. After endotracheal intubation, he was brought to our emergency department where his Glasgow Coma Scale score was 3T. His pulse ranged from 120 to 140 and his blood pressure from 120 to 160 systolic to 80 to 90 diastolic. His temperature was 98.6°F, and pulse oximetry was 99% on room air with an endotracheal tube in place. External injuries were limited to a 1-cm full-thickness skin burn over the medial left foot at the base of the first phalanx and a 3-cm full-thickness skin burn over the lateral right foot, considered to be exit wounds. He was placed under a cooling protocol for 48 hours, paralyzed with cisatracurium, and sedated with propofol and fentanyl. On day 4, when sedation was discontinued he was agitated and unresponsive to voice or painful stimuli. Pupils were of normal size and reactivity. Deep tendon reflex examination evoked myoclonus. Tone was increased in the lower extremities. Plantar reflexes were extensor bilaterally. Head and spine CT performed on day 1 and day 4 was normal. On day 5, brain MRI revealed hyperintense signal and parenchymal thickening (loss of gray-white distinction and sulcal narrowing) in the mesial occipital regions J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 107 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Kamyar and Trobe bilaterally on T2, FLAIR, and diffusion sequences and hypointense signal in the corresponding regions on appar-ent diffusion coefficient maps (Fig. 1). There were no other unequivocal abnormalities. These findings suggested rela-tively selective ischemia of the mesial occipital lobes. In the next 3 weeks, the patient's agitation gradually dissipated, and he became more alert. Once able to communicate, he expressed an inability to see. Our examination on day 25 disclosed that he was attentive and oriented to location but not to time. He could not recall what he had eaten earlier that day. Cranial nerve examination was normal except that he had only hand movement vision in both eyes. He had normal strength, tone, reflexes, and sensation in all extremities. We made a diagnosis of cardiogenic hypoxic-ischemic encephalop-athy most prominently affecting the mesial occipital lobes. By day 50, visual acuity had improved to 20/200 in each eye. Humphrey visual field examination showed severely constricted fields (Fig. 2) in a pattern suggestive of bilateral homonymous hemianopia with macular sparing. By day 76, visual acuity had improved to 20/30 in both eyes, but visual fields were unchanged. On informal mental status testing, he appeared to be intact. DISCUSSION Our patient developed bilateral homonymous hemi-anopias with macular sparing in the setting of electrical shock. Although other parts of the brain were clinically affected, the most enduring deficit was cerebral visual loss. The structural imaging abnormalities were strikingly limi-ted to the mesial occipital region. We believe that the visual loss was not a direct result of current flow to the brain, but an indirect effect of cardiogenic hypotension caused by electrical effects on the cardiac conduction system. Oddly, there has been only one report in which cardiogenic hypotension was documented before cerebral visual loss in the setting of electrical injury (3). Most electrical injury to the body, including that to the visual system, has been attributed to heat damage. The principal mediator of tissue damage in electrical injury is the conversion of electrical energy into heat. The amount of heat generated is dependent on the amount and duration of current flow and the amount of resistance encountered by the current in the various body tissues. As it searches for a grounding source, the electrical current typically takes the path of least resistance. Tissues with the least resistance include nerves and blood vessels. Tissues with greater resistance include muscle, skin, tendon, fat, and bone (5). When current exits the body, a significant amount of thermal damage may cause full-thickness skin burns, as occurred in our patient's feet. The familiar ophthalmic manifestations of electrical injury are generally thought to be caused by the thermal effect of the electrical current passing through the eyes (6). The most commonly described ocular injuries are corneal burns (7), cataracts (8,9), subretinal macular hemorrhages (10), bilateral macular holes and cysts (11,12), and anterior ischemic optic neuropathy (6). The mechanism of cerebral visual loss has received less attention. A logical mechanism of injury would be direct thermal injury to the skull or brain. Indeed, Tamler (1) reported a 36-year-old man shocked by a high-tension line carrying 12,000 V who had a right occipital region scalp burn and a congruous left homonymous hemianopia attributed to direct injury from the burn (1). This report antedated the availability of sophisticated brain imaging. Patel and Lo (2) reported a 31-year-old man who was shocked when his right hand came into contact with a 15,000-V capacitor. Cerebral angiography disclosed cere-bral thrombosis in the distal left vein of Labbe´, which caused right facial and arm numbness and weakness and a ‘‘right visual field defect.'' No further clinical details were provided (2). The authors proposed that the venous thrombosis developed from vasospasm and intimal damage induced by the heating effect of electricity that traveled through brain vessels. Gans and Glaser (4) reported a 53-year-old hyper-tensive man who was shocked in the left hand while working with a set of wires carrying 220 V of AC (4). He suffered no immediate deleterious effects, but awakened 4 days later with the inability to see objects in his right hemifield. CT revealed a hypodense lesion in the left occipital region consistent with a cortical infarct. Although the authors proposed a causal relationship between the occipital infarct and the electric injury, there is scant evidence to support that contention. More pertinent to our case is the report of Perez- Molina et al (3), who described a 16-year-old boy who experienced an electrical injury while playing an electric guitar (3). He had a cardiopulmonary arrest and was resuscitated after 10 minutes. Upon awakening, he complained of an inability to see. FLAIR MRI the next day revealed hyperintense signal in both occipital lobes. There was no mention of a diffusion sequence. The MRI findings resolved after 20 days, as did the patient's visual loss. Because of the reversibility of the patient's condition, the authors attributed the injury to a direct electrical injury to the brain. They proposed the phenomenon of ‘‘electro-poration'' or alteration in the electrochemical balance between the intracellular and extracellular compartments because of the effect of electricity on cellular plasma membranes. This phenomenon can increase membrane porosity and result in an increase in extracellular edema. The authors attributed the transient blindness and MRI 108 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Electrical Shock J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 FIG. 1. Brain MRI performed on day 5 after the electrical shock episode. Axial sections through the medial occipital regions (left column) show high signal on the T2 (A) and diffusion (B) images and low signal on the corresponding apparent diffusion coefficient (ADC) map (C). The T2, diffusion, and ADC axial images in the parietal region (right column) are normal. 109 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro-Ophthalmol, Vol. 29, No. 2, 2009 Kamyar and Trobe FIG. 2. Humphrey visual field examination on day 50 shows macular-sparing bilateral homonymous hemianopias. abnormalities to vasogenic edema similar to that seen in posterior reversible encephalopathy syndrome (PRES). We suspect that the patient reported by Perez-Molina et al (3) experienced visual loss from the same mechanism as did our patient but in milder form. Cardiopulmonary arrest is the most common cause of immediate death in electrical injury (13). If there is successful resuscitation after electrical injury, the patient's outcome usually depends on whether hypoxic-ischemic encephalopathy develops. Although cardiopulmonary arrest is a common outcome of severe electrical injury, there is no previously reported link between cerebral visual loss and hypoxic-ischemic encephalopathy induced by electrical injury. It is possible that this connection has been overlooked because clinicians and radiologists expect systemic hypotension to cause infarction in the ‘‘water-shed'' domain that lies between the major cerebral arteries. The watershed domain in the posterior brain hemispheres is classically in the parieto-occipital region rather than in the mesial occipital region, the area affected in our patient. The clinical correlate of parieto-occipital ischemia is Balint- Holmes syndrome, in which patients typically have visual spatial, attentional, and ocular motor deficits with sparing of visual acuity and visual fields (14). However, several reports have documented that the epicenter of cerebral damage in systemic hypotension may be the mesial occip-ital region or primary visual cortex (15). The clinical correlate of mesial occipital damage is bilateral homony-mous hemianopia, often with macular sparing, as our patient demonstrated. The macular sparing that often accompanies bilateral homonymous hemianopias in such cases has been attributed to the fact that the occipital polar region that serves central (‘‘macular'') vision receives a dual blood supply, being served by both the posterior and middle cerebral arteries (14,15). REFERENCES 1. Tamler E. Electric cataract. Am J Ophthalmol 1962;54:865-6. 2. Patel A, Lo R. Electrical injury with cerebral venous thrombosis: case report and review of the literature. Stroke 1993;24:903-5. 3. Perez-Molina JM, Velazquez-Perez B, Mondejar-Marin S, et al. Secuelas neurologicas tras electrocucuion: presentacion de un caso y revsion de la bibliografia. Rev Neurol 2006;43:610-2. 4. Gans M, Glaser JS. Homonymous hemianopia following electrical injury. J Clin Neuroophthal 1986;6:218-21. 5. Duff K, McCaffrey RJ. Electrical injury and lightning injury: a review of their mechanisms and neuropsychological, psychiatric, and neurological sequelae. Neuropsychol Rev 2001;11:101-15. 6. Grover S, Goodwin J. Lightning and electrical injuries: neuro-ophthalmologic aspects. Semin Neurol 1995;15:335-41. 7. Borderie V. Clinical aspects of corneal burns (in French). J Fr Ophtalmol 2004;27:1170-4. 8. Grewal DS, Jain R, Brar GS, et al. Unilateral electric cataract: Scheimpflug imaging and review of the literature. J Cataract Refract Surg 2007;33:1116-9. 9. Seth RK, Abedi G, Daccache AJ, et al. Cataract secondary to electrical shock from a Taser gun. J Cataract Refract Surg 2007;33: 1664-5. 10. Liyanage SE, Khemka S, de Alwis DV. Acute subretinal macular haemorrhage following an accidental electrical shock. Eye 2006;20: 1422-4. 11. Espaillat A, Janigian R Jr, To K. Cataracts, bilateral macular holes, and rhegmatogenous retinal detachment induced by lightning. Am J Ophthalmol 1999;127:216-7. 12. Manrique-Cerrillo M, Murillo-Lopez S, Leizaola-Fernandez C, et al. Bilateral macular cysts secondary to electric current strike: a case report (in Spanish). Arch Soc Esp Oftalmol 2004;79:37-9. 13. Fontanarosa PB. Electrical shock and lightning strike. Ann Emerg Med 1993;22:378-87. 14. Trobe JD. The Neurology of Vision. New York: Oxford University Press; 2001:310-2. 15. Margolin E, Gujar SK, Trobe JD. Isolated cortical visual loss with subtle brain MRI abnormalities in a case of hypoxic-ischemic encephalopathy. J Neuroophthalmol 2007;27:292-6. 110 © 2009 Lippincott Williams & Wilkins Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. |