| Description |
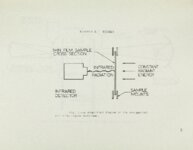
A new, high heating rate, thermoanalytical technique for the study of condensed phase reactions was developed and applied in an investigation of a number of state-of-the-art binder-fuels and ammonium perchlorate based, propellant-like materials. The polymers used in the binders were polybutadiene-acrylic acid, polybutadiene-acrylonitrile, polybutadiene (carboxy terminated), polybutadiene (hydroxy terminated), polyisobutylene, polyurethane and fluorocarbon polymer. Heating rates of 50oK/sec to 300oK/sec were used in the study. The experimental method permitted detection and quantitative measurement of endothermic and/or exothermic energy effects. The species and pressure of the environmental gas were controlled, and tests in both oxygen and nitrogen were conducted at pressures ranging from 0.02 to 6.85 atmospheres. The samples were prepared in the form of thin films, 100 to 300 microns thick and 1.5 cm in diameter, which were supported only at their outer edges. Constant, uniform radiant energy impinging on one side of the film heated the sample, and the temperature of the opposite side was monitored by a rapid response infrared detector. Even at the high heating rates employed, the temperature difference across films was less than 10°C. The detector output was digitized, converted into time temperature results by use of calibration data, and reduced to a form similar to conventional thermal analysis results. The data are reported as differential thermograms which were formed by differencing the experimental thermograms with the analytical thermograms of a hypothetical inert sample of the same thermal properties as the test film. |